Beskæftigelsesudvalget 2019-20
BEU Alm.del Bilag 101
Offentligt
Carbon
nanotubes:
Scientific basis
for setting
a health-based
occupational
exposure limit
Sarah Søs Poulsen, Nicklas Raun Jacobsen, Niels Hadrup,
Karin Sørig Hougaard, Anne Thoustrup Saber and Ulla Vogel
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
CARBON NANOTUBES: SCIENTIFIC BASIS FOR
SETTING A HEALTH-BASED OCCUPATIONAL
EXPOSURE LIMIT
Sarah Søs Poulsen
Nicklas Raun Jacobsen
Niels Hadrup
Karin Sørig Hougaard
Anne Thoustrup Saber
Ulla Vogel
Det Nationale Forskningscenter for Arbejdsmiljø, København 2018
1
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
NFA-report
Title
Carbon nanotubes: Scientific basis for setting a health-based occupational
exposure limit
Sarah Søs Poulsen, Nicklas Raun Jacobsen, Niels Hadrup, Karin Sørig
Hougaard, Anne Thoustrup Saber and Ulla Vogel
The National Research Centre for the Working Environment (NFA)
The National Research Centre for the Working Environment (NFA)
September 2018
978-87-7904-350-3
nfa.dk
Authors
Institution
Publisher
Published
ISBN
Internet version
The National Research Centre for the Working Environment (NFA)
Lersø Parkallé 105
DK-2100 Copenhagen
Tlf.: +45 39165200
Fax: +45 39165201
e-mail: nfa@nfa.dk
Website:
nfa.dk
2
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
F
OREWORD
In 2015, the Danish Working Environment Council made 22 recommendations to
promote safe handling of nanomaterials in the working environment, which were
enforced by the Minister of Employment. One of these recommendations was ‘That the
Danish Working Environment Authority in cooperation with relevant scientific experts
assesses whether adequate scientific documentation can be provided to use the scientific
quality committee for an assessment of the scientific evidence to determine limit values
for specific nanomaterials in the work environment.’ (https://www.amr.dk/nano.aspx).
On this background, The Danish Working Environment Authority asked the National
Research Centre for the Working Environment to review the scientific evidence with the
aim of clarifying the possibilities for suggesting nanospecific occupational exposure
limits for three different nanomaterials (titanium dioxide, carbon black and carbon
nanotubes).
The purpose of the present report is to suggest a health-based occupational exposure
limit for carbon nanotubes.
Elizabeth Bengtsen and Karen Bo Frydendall, National Research Centre for the Working
Environment, are gratefully acknowledged for assistance with literature search.
The working group wishes to thank Chief Toxicologist Poul Bo Larsen, DHI, Denmark,
for reviewing the report.
Copenhagen, August 2018
3
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
E
XECUTIVE SUMMARY
In this report, a working group at the National Research Centre for the Working
Environment reviews data relevant to assessing the hazard of carbon nanotubes (CNTs),
i.e. human studies (Chapter 2), toxicokinetics (Chapter 3), animal studies (Chapter 4),
mechanisms of toxicity (Chapter 5), previous risk assessments of CNTs (Chapter 6),
scientific basis for setting an OEL (Chapter 7) and finally summarize and suggest a
health-based occupational exposure limit for CNTs (Chapter 8). The focus of this report
is only occupational exposure by inhalation.
Carbon nanotubes are a very diverse class of nanomaterials with large variation in
physico-chemical properties including diameter, length, specific surface area, level and
type of contaminations, and surface modifications. Variation in toxicity potential based
on different physico-chemical properties has been reported, but at the time of this report,
the relationship between physico-chemical properties of CNTs and their inhalation
toxicity is not fully clarified. Furthermore, most commercially available CNT
preparations are very heterogeneous. Therefore, the present working group considers
toxicity data from all types of CNTs in order to obtain a precautious approach and
derivation of an OEL value that protects against as many different types of CNTs as
possible.
Assessments of human occupational exposure to CNTs at settings such as laboratories
and production sites have reported personal breathing zone concentration levels ranging
from non-detectable to ca. 80 µg/m
3
. This demonstrates that human occupational
exposure does occur during handling of CNTs. However, at present almost no human
data on toxicity and epidemiological studies is available. The current working group
therefore used studies in mice and rats to assess potential human hazard. Inhalation
studies were prioritized and risk assessments were solely based on these. However, for
the description of toxicological endpoints and mechanism of toxicity, studies using
pulmonary deposition from intratracheal instillation exposure and pharyngeal aspiration
exposure were included when no quality inhalation studies were available.
Pulmonary inflammation, and inflammatory-related changes, was the most commonly
reported adverse effect of pulmonary exposure to CNTs. Four sub-chronic and one
chronic study inhalation study in rats were identified as suitable for identification if
relevant NOAECs/ LOAECs and determining a derived-no-effect level (DNEL) for
pulmonary inflammation. In general, these studies identified ”no observed adverse
effect concentrations”(NOAECs) ranging from 0.05 mg/m
3
to 1 mg/m
3
and ”lowest
observed adverse effect concentrations” (LOAECs) ranging from 0.25 mg/m
3
to 5 mg/m
3
.
The deposited surface area of the CNTs was identified as a predictor of pulmonary
inflammation (neutrophil influx in the broncho alveolar lavage fluid). As dose-
dependency was identified for inflammation and as it was possible to detect a NOAEC,
inflammation was considered a threshold effect.
The genotoxic and carcinogenic potential of CNTs were investigated in several studies.
CNT-induced genotoxicity was reported; however, although diameter thickness was
suggested as a driver of CNT-induced genotoxicity, no clear coupling to physico-
4
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
chemical properties could be identified. In addition, no dose response relationship was
identified regarding the genotoxic properties. One chronic cancer study in rats was
identified as suitable for risk assessment. This 2 year inhalation study investigated
pulmonary pathological changes after exposure to the long and thick MWCNT type
called MWNT-7/XNRI-7, and the authors reported lung adenomas and carcinomas at the
middle and high dose (0.2 and 2 mg/m
3
, 6 h/day, 5 days/week for 104 weeks), whereas
0.02 mg/m
3
was found as a NOAEC. The same MWCNT type (MWNT-7/XNRI-7) had
previously been classified as possibly carcinogenic by IARC. Dose-response relationships
have been identified for MWCNT-induced carcinogenic effects in several independent
studies. The present working group found that the mechanism of action of CNT-induced
carcinogenic effect has not been fully clarified. CNTs have reported to induce ROS
generation similar to carbon black. CNTs may also induce genotoxicity through they
fibrous shape, both in regards to diameter thickness and length. In addition, secondary
genotoxicity due to CNT-induced inflammation has been recognized as an important
and well-documented mechanism of action for the development of lung cancer. Based on
the lack of dose-response relationship for genotoxicity and the unclear mode of action for
cancer, the current working group did not find sufficient evidence for a threshold
mechanism for CNT-induced carcinogenicity and decided to consider it as non-threshold
effect.
CNT-induced cardiovascular effects were reported in several animal studies. Both
primary changes, such as accelerated plaque progression, and changes related to/or
leading to cardiovascular effects, such as the acute phase response, were identified.
Dose-response relationships have only sparsely been reported for CNT-induced
increased plaque progression, whereas dose-response relationships have been
established between CNT exposure and increased levels of acute phase response
proteins. CNT-induced atherosclerotic effects have solely used pulmonary deposition as
exposure method, and thus, the studies cannot be used to establish OELs. Due to the
close interplay between inflammation, acute phase response and plaque progression, the
current working group regards inflammation as a proxy for cardiovascular effects.
Cardiovascular effects are considered a threshold effects that is regulated in parallel to
inflammation.
The present working group regards inflammation and carcinogenicity as the critical
adverse effects of CNT exposure by inhalation and the subsequent risk assessments are
conducted based on studies reporting these effects. Based on dose-response relationships
and mode of action for these effects, the current working group decided to perform the
risk assessment based on both a threshold and a non-threshold mechanism of action.
Four sub-chronic and one chronic inhalation study in rats were identified as suitable for
determining a DNEL for pulmonary inflammation. A conservation approach was
selected and the DNEL was calculated based on the study using the CNT with the largest
specific surface area and reporting the lowest NOAEC estimate. The suggested exposure
limit based on inflammation was 1 µg/m
3
.
For the non-threshold approach on carcinogenic effects, the 2 year inhalation study in
rats were identified as suitable and excess cancer risks at the levels of 1:1,000, 1:10,000
and 1 in 100,000 were calculated based this study using two approaches (please see the
5
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
accompanying table). In the first approach, lung burden was used to estimate the
exposure levels. In the second approach, air concentrations were used directly.
Independently of the applied method for risk assessment, the acceptable exposure levels
were all very low. These levels are all more than 5 magnitudes lower than the present
Danish occupational exposure limit for bulk carbon black of 3.5 mg/m
3
.
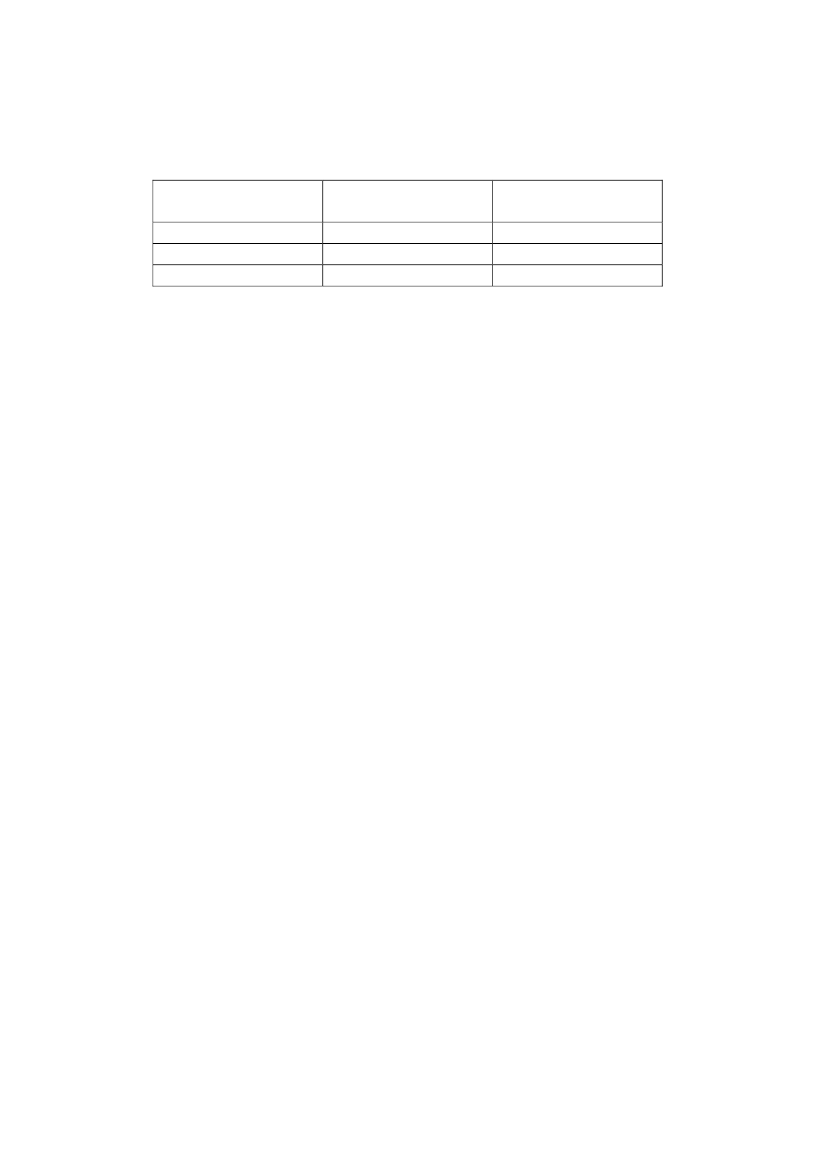
Suggestion for an OEL for CNTs
Lung cancer
Lung cancer
Mechanism of action
Inflammation
(Method I)
(Method II)
Threshold based
DNEL
1 µg/m
3
Non-threshold based Excess cancer risk:
1:1,000
0.03 µg/m
3
0.043 µg/m
3
1:10,000
0.003 µg/m
3
0.0043 µg/m
3
1:100,000
0.0003 µg/m
3
0.00043 µg/m
3
Table showing overview of DNEL based on a threshold based mechanism of action
and exposure levels resulting in excess cancer risk levels at 1:1000, 1:10 000 and 1: 100
000 based on a non-threshold based mechanism of action.
The present working group regards cancer as the most critical adverse effect of CNT
inhalation and recommends the approach using the excess lung cancer risk estimates
based on lung burden, since this approach takes the retained pulmonary dose into
account. Thus, the expected excess lung cancer risk based on lung burden approach is
1:1,000 at 0.03 µg/m
3
, 1:10,000 at 0.003 µg/m
3
and 1:100,000 at 0.0003 µg/m
3
.
6
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
D
ANSK SAMMENFATNING
I denne rapport vurderer en arbejdsgruppe ved Det Nationale Forskningscenter for
Arbejdsmiljø data, der er relevante for at vurdere faren ved eksponering for
kulstofnanorør (CNTer), dvs. humane studier (kapitel 2), toksikokinetik (kapitel 3),
dyreforsøg (kapitel 4), toksicitetsmekanismer (kapitel 5), tidligere risikovurderinger af
CNTer (kapitel 6), videnskabeligt grundlag for fastlæggelse af en grænseværdi i
arbejdsmiljøet (kapitel 7) og endelig opsummeres og foreslås en helbredsbaseret
grænseværdi for CNTer i arbejdsmiljøet (kapitel 8). Fokus i denne rapport er alene på
erhvervsmæssig eksponering ved indånding.
Kulstofnanorør er en meget forskelligartet gruppe af nanomaterialer med store
variationer i de fysisk-kemiske egenskaber. Disse inkluderer diameter, størrelse, længde,
specifikt overfladeareal, mængde og type af metalurenheder og overflademodifikationer.
Variationer i CNTers fysisk-kemiske egenskaber har vist sig at kunne påvirke det
toksiske potentiale, men på nuværende tidspunkt er sammenhænge mellem CNTers
fysisk-kemiske egenskaber og deres toksicitet efter indånding endnu ikke fuldstændigt
klarlagt. Derudover er de fleste kommercielt tilgængelige CNT præparationer meget
heterogene af natur. Derfor anser den nærværende arbejdsgruppe alle CNT typer som
farlige ved indånding og foreslår at regulere alle CNTer som én gruppe.
Eksponeringsmålinger på arbejdspladser, såsom laboratorier og
produktionsvirksomheder, har påvist CNT koncentrationer i den personlige
indåndingszone som spænder fra under detektionsgrænsen til ca. 80 µg/m
3
. Dette viser,
at der er erhvervsmæssig eksponering for CNT ved håndteringen af CNT på
arbejdspladser. Men på nuværende tidspunkt forefindes der stort set ingen data på
toksicitet i mennesker eller epidemiologiske studier. Den nærværende arbejdsgruppe har
derfor brugt studier i mus og rotter til at vurdere den potentielle menneskelige
helbredsrisiko. Subkroniske og kroniske inhalationsstudier blev prioriteret og
risikovurderingerne blev udelukkende foretaget på baggrund af disse. Dog blev studier,
der anvendte lungedeponering ved intratracheal instillation, inkluderet til beskrivelse af
toksikologiske endepunkter og toksisitetsmekanismer, når der ikke forelå
inhalationsstudier af tilstrækkelig kvalitet.
Lungeinflammation, og inflammationsrelaterede ændringer var de hyppigst
rapporterede helbredseffekter efter lungeeksponering af CNTer. Fire sub-kroniske og et
kronisk inhalationsstudie i rotter blev fundet egnede til at bestemme en ”derived-no-
effect level” (DNEL) for lungeinflammation. Disse studier rapporterede generelt ”no
observed adverse effect concentrations” (NOAECs)mellem 0.05 mg/m
3
og 1 mg/m
3
og
”lowest observed adverse effect concentrations” (LOAECs) mellem 0.25 mg/m
3
og 5
mg/m
3
. Det deponerede overfladeareal af CNTer blev identificeret som en prædiktor for
lungeinflammation (neutrofilt influx i lungeskyllevæsken). Der var dosisafhængighed
for CNT-induceret lungeinflammation, og da det var muligt at bestemme en NOAEC,
blev inflammation anset for at være en tærskeleffekt.
CNTers genotoksiske og kræftfremkaldende potentialer er blevet undersøgt i flere
studier. CNT-induceret genotoksicitet er blevet rapporteret, men selvom
7
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
diametertykkelse er blevet forslået som prædiktor for CNT-induceret genotoksicitet, er
der endnu ikke fundet en klar sammenhæng mellem genotoksicitet og de fysisk-kemiske
egenskaber for CNTer. Derudover blev der ikke fundet dosis-respons sammenhæng.
Et kronisk kræftstudie i rotter blev identificeret som egnet til risikovurdering. I dette 2
års inhalationsstudie blev patologiske ændringer i lungen efter eksponering for den
lange og tykke CNT type kaldet MWNT-7/XNRI-7 undersøgt, og forfatterne
rapporterede lungekræft ved den midterste og højeste dosis (0,2 og 2 mg/m
3
, 6
timer/dag, 5 dage/uge i 104 uger). Denne type CNT (MWNT-7/XNRI-7) er blevet
klassificeret som muligvis kræftfremkaldende (2b) af IARC. Der blev identificeret
dosisafhængighed for CNT-induceret kræft i flere uafhængige studier. Den nærværende
arbejdsgruppe fandt ikke, at mekanismen for CNT-induceret kræft er fuldstændig
klarlagt. Studier har vist, at CNTer kan inducere reaktive oxygen-forbindelser på samme
måde som carbon black. De kan muligvis også inducere genotoksicitet via deres
fiberform, både i forhold til diameter og til længde. Derudover er det velkendt at
sekundær genotoksicitet, pga. CNT-induceret inflammation, er en mekanisme for
udviklingen af kræft. På baggrund af den manglende dosisafhængighed for
genotoksicitet og en uklar virkningsmekanisme for kræft, fandt den nærværende
arbejdsgruppe ikke tilstrækkelig bevis for at CNT-induceret kræft er en tærskeleffekt.
CNT-induceret kræft blev derfor anset som en ikke-tærskeleffekt.
Resultaterne fra flere dyrestudier viste CNT-inducerede effekter på hjerte-karsystemet.
Dette gjaldt både primære effekter, som åreforkalkning, og ændringer relateret til eller
førende til hjerte-kareffekter, så som akutfaseresponset. Dosisafhængighed er kun i
begrænset omfang beskrevet for CNT-induceret øget åreforkalkning, hvorimod
dosisafhængighed er veldokumenteret for sammenhængen mellem CNT eksponering og
akutfaseproteiner. Men da de studier, hvor resultaterne viser CNT-inducerede effekter
på hjerte-karsystemet, udelukkende har brugt lungedeponering som
eksponeringsmetode, kan de ikke bruges til risikovurdering af CNTer. På grund af den
tætte sammenhæng mellem inflammation, akutfaseresponset og åreforkalkning har den
nærværende arbejdsgruppe valgt at anse inflammation som en proxy for effekter på
hjerte-karsystemet. Hjerte-kareffekter blev derfor anset som en tærskeleffekt på lige fod
med inflammation.
Den nærværende arbejdsgruppe anser inflammation og carcinogenicitet som de vigtigste
negative helbredseffekter forårsaget af indånding af CNT, og de efterfølgende
risikovurderinger er baseret på studier, der rapporterer disse effekter. Baseret på viden
om dosis-respons sammenhæng og underliggende biologiske mekanismer har den
nærværende arbejdsgruppe besluttet både at foretage risikovurderinger baseret på en
tærskeleffekt og på en ikke-tærskeleffekt. Fire subkroniske og et kronisk
inhalationsstudie i rotter blev identificeret som velegnede til fastlæggelse af DNEL for
lungeinflammation. Den nærværende arbejdsgruppe valgte en konservativ tilgang, og
derfor blev DNEL beregnet med udgangspunkt i det studie, som anvendte den CNT, der
havde det største specifikke overfladeareal, og som rapporterede det laveste NOAEC.
Den foreslåede grænseværdi for kulstofnanorør baseret på inflammation er 1 µg/m
3
.
Den nærværende arbejdsgruppe valgte at anvende ikke-tærskeleffekt tilgangen til
beregning af grænseværdi baseret på kræftrisiko, og et 2-års inhalationsstudie i rotter
8
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
blev identificeret som velegnet. De eksponeringsniveauer, som resulterer i overskydende
kræftrisiko hos 1:1.000, 1:10.000 og 1 ud af 100.000 udsatte, blev beregnet på baggrund af
dette studie på to forskellige måder og er vist i tabellen nedenfor. Ved den første
beregningsmetode bruges den lunge-deponerede dosis til at estimere det tilsvarende
eksponeringsniveau. Ved den anden beregningsmetode blev luftkoncentrationer
anvendt direkte. Uafhængigt af metodevalg er alle de beregnede grænseværdier for
kulstofnanorør meget lave. Grænseværdierne er alle mere end 5 000 gange lavere end
den nuværende danske grænseværdi for carbon black (som er 3,5 mg/m
3
).
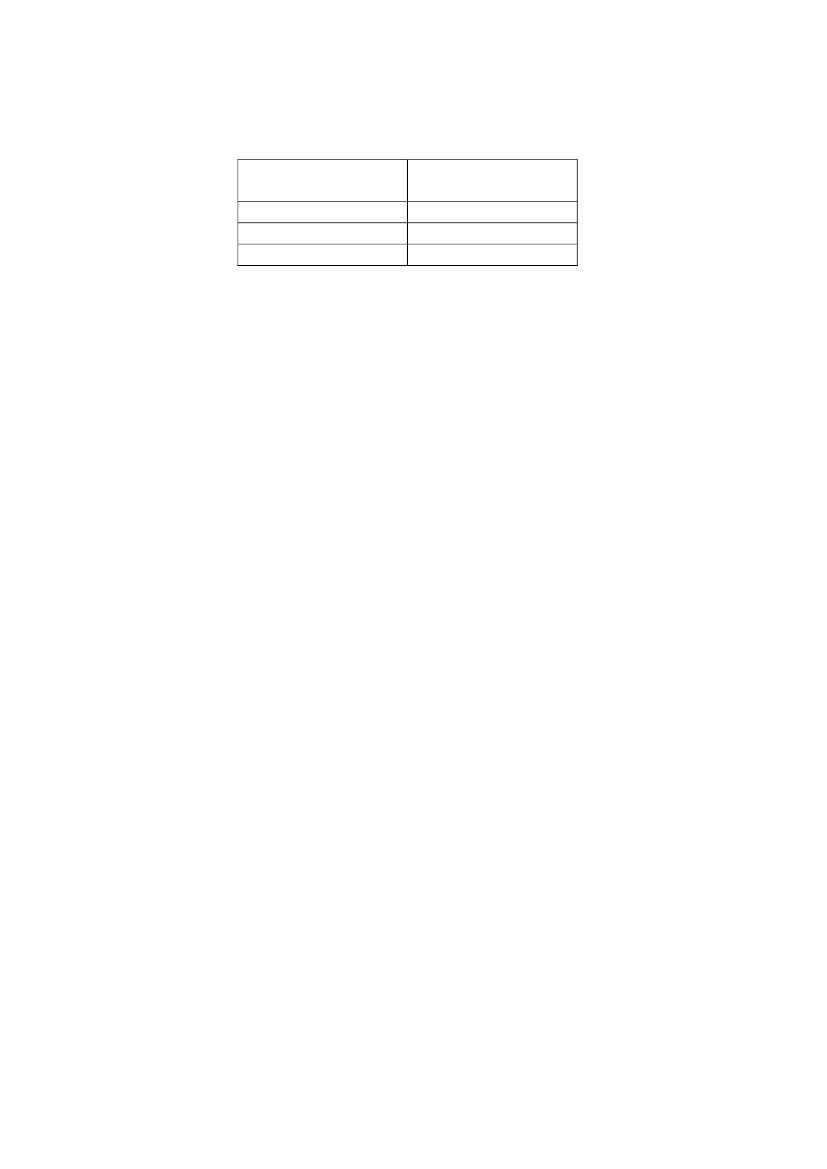
Forslag til grænseværdi for kulstofnanorør
Lungekræft
Lungekræft
Virkningsmekanisme
Inflammation
(Metode I)
(Metode II)
Tærskeleffekt-baseret DNEL
1 µg/m
3
Ikke-tærskeleffekt-
Overskydende
baseret
lungekræft:
1:1.000
0,03 µg/m
3
0,043 µg/m
3
1:10.000
0,003 µg/m
3
0,0043 µg/m
3
1:100.000
0,0003 µg/m
3
0,00043 µg/m
3
Tabellen viser en oversigt over DNEL baseret på tærskeleffekt som
virkningsmekanisme for lungeinflammation og de eksponeringsniveauer, som
resulterer i overskydende kræftrisiko hos 1:1.000, 1:10.000 og 1 ud af 100.000 baseret på
en ikke-tærskeleffekt-baseret biologisk virkningsmekanisme for lungekræft.
Den nærværende arbejdsgruppe anser kræft for at være den vigtigste helbredseffekt ved
indånding af kulstofnanorør og anbefaler ydermere at bruge beregningsmetode I fordi
denne beregningsmetode tager udgangspunkt i den faktiske lungedeponerede dosis.
Det estimeres derfor at 0.03 µg/m
3
kulstofnanorør vil forårsage 1:1.000 overskydende
lungekræfttilfælde ved indånding i arbejdsmiljøet, mens 0,003 µg/m
3
kulstofnanorør vil
forårsage 1:10.000 overskydende lungekræfttilfælde og 0,0003 µg/m
3
forventes at
forårsage 1:100,000 overskydende lungekræfttilfælde.
9
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
C
ONTENTS
Foreword ........................................................................................................................................ 3
Executive summary ....................................................................................................................... 4
Dansk sammenfatning .................................................................................................................. 7
Contents ........................................................................................................................................ 10
Abbreviations ............................................................................................................................... 11
Introduction.................................................................................................................................. 12
Human studies............................................................................................................................. 15
Exposure ................................................................................................................................... 15
Biomonitoring .......................................................................................................................... 18
Toxicokinetics .............................................................................................................................. 19
Animal studies ............................................................................................................................. 21
Rodent versus human response ............................................................................................ 21
Intratracheal instillation versus inhalation .......................................................................... 21
Selection of studies and endpoints ....................................................................................... 22
Pulmonary inflammation ....................................................................................................... 22
Genotoxicity and cancer ......................................................................................................... 26
Cardiovascular effects............................................................................................................. 29
Reprotoxicity ............................................................................................................................ 31
Mechanisms of toxicity ............................................................................................................... 32
Pulmonary inflammation ....................................................................................................... 32
Genotoxicity and cancer ......................................................................................................... 33
Cardiovascular effects............................................................................................................. 34
Dose-response relationships .................................................................................................. 35
Previous risk assessments of carbon nanotubes ..................................................................... 37
Aschberger et al. 2010 ............................................................................................................. 37
Pauluhn 2010 ............................................................................................................................ 37
ENRHES.................................................................................................................................... 38
NIOSH....................................................................................................................................... 38
IARC .......................................................................................................................................... 38
Scientific basis for an occupational exposure limit ................................................................. 41
Calculations of exposure limits based on cancer as non-threshold effect ....................... 41
Calculations of exposure limits based on inflammation as threshold effect .................. 44
Conclusion .................................................................................................................................... 46
References ..................................................................................................................................... 48
10
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
A
BBREVIATIONS
ALP
BAL
BALF
BET
CVD
CNT
CRP
DNEL
DSP
DWCNT
EC
ECHA
ENRHES
EM
GI
IARC
ICAM
INEL
IP
HARN
HDL
HiPCO
LDH
LDL
LOAEC
MWCNT
NIOSH
NM
NOAEC
OEL
OECD
REACH
REL
SAA
SWCNT
TEM
TWA
VCAM
Alkaline phosphatase
Broncho alveolar lavage
Broncho alveolar lavage fluid
Brunauer–Emmett–Teller
Cardiovascular disease
Carbon nanotube
C-reactive protein
Derived-no-Effect Level
Daily sperm production
Double-walled carbon nanotubes
Elemental carbon
European Chemicals Agency
Engineered Nanoparticles: Review of Health and Environmental Safety
Electron microscopy
Gastrointestinal
The International Agency for Research on Cancer
Intercellular Adhesion Molecule
Human indicative no-effect levels
Intraperitoneal
High aspect ratio nanomaterials
High-density lipoproteins
High-pressure carbon monoxide method
Lactate dehydrogenase
Low-density lipoproteins
Lowest observed adverse effect concentration
Multi-walled carbon nanotube
National Institute for Occupational Safety and Health
Nanomaterial
No observed adverse effect concentration
Occupational exposure limit
Organisation for Economic Co-operation and Development
Registration, Evaluation, Authorisation and Restriction of Chemicals
Recommended exposure limit
Serum amyloid A
Single-walled carbon nanotube
Transmission electron microscopy
Time-weighted average
Vascular cell adhesion molecule
11
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
I
NTRODUCTION
Carbon nanotubes (CNTs) comprise a group of poorly soluble, highly variable,
cylindrical, hollow, fibrous nanomaterials. They are grouped according to their number
of side-walls and generally divided into three categories: Single-walled (SW), double-
walled (DW), or multi-walled (MW) CNTs. Their side-walls are made of carbon atoms,
mainly in sp
2
configuration, arranged in inter-connected hexagon similar to that of
graphene sheets. As the name implies, SWCNTs consist of one rolled-up graphene layer,
whereas DW- and MWCNTs consist of two or more layers. The difference in wall
numbers affects the diameter and rigidity of the CNTs; whereas some are entangled,
others are fiber-like. Correspondingly, the diameters can vary from around 1 nm (most
SWCNTs) to up to 150 µm (some MWCNTs) (Jensen et al. 2015). Because of their long
lengths (up to several mm), CNTs are high aspect ratio (length:diameter) nanomaterials
(HARN). Long CNTs (over 15 µm in length) comply with the WHO fiber paradigm,
which states that fibers with long lengths, small diameters and high biopersistence
display increased toxicity, as they reach the alveoli region of the lungs, retain their
structure, and are difficult for the macrophages to phagocytize (Donaldson et al. 2010).
The physical appearance of CNTs can vary greatly from one type of CNT to another. The
rigidity of the CNT, and thus its level of fiber-like appearance, relies prominently on its
number of graphene walls. However, MWCNTs with few walls resembles SWCNT
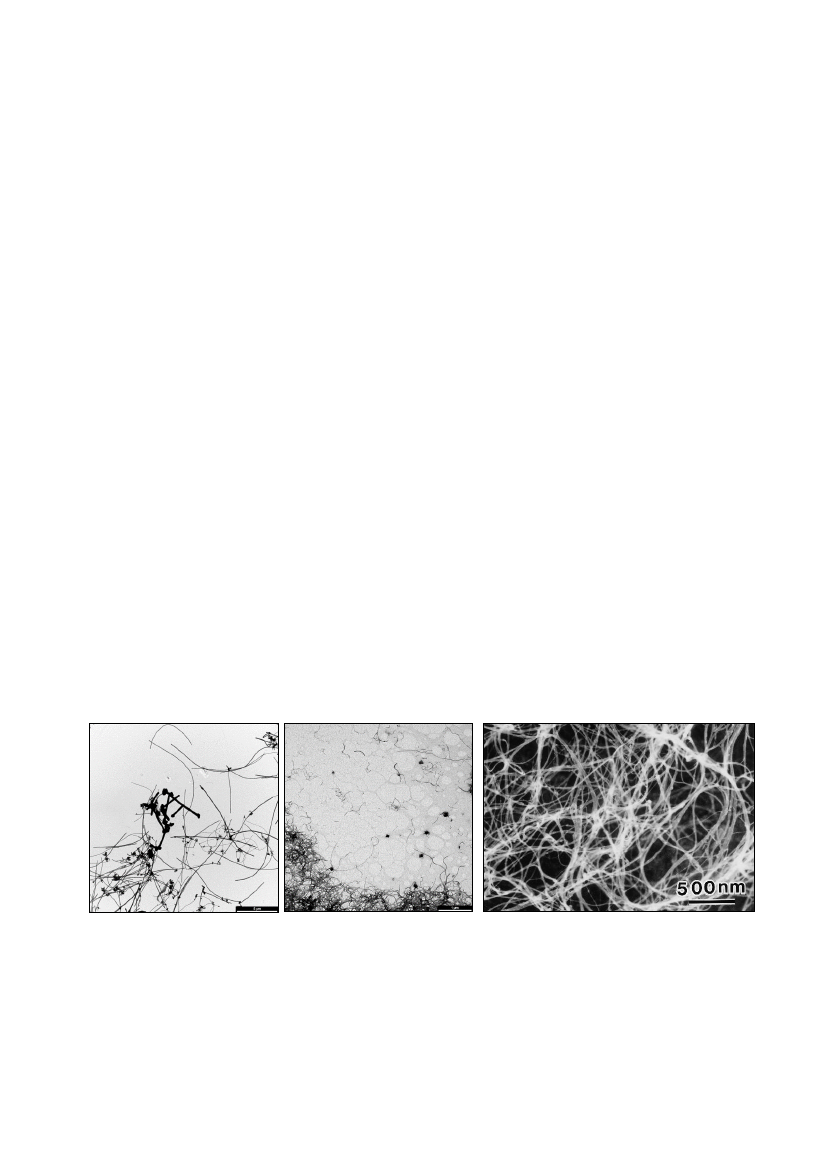
physically more than fiber-like MWNCTs with several walls (Figure 1). It is therefore
difficult to address specific SW- or MWCNT effects. Instead of separating CNTs based on
wall numbers, the current working group has chosen to separating based on physico-
chemical properties as surface area, length, diameter, chemical composition etc. This
report therefore does not distinguish between SW- or MWCNT effects, and CNTs data
from both SWCNT and MWCNT were evaluated. Based on this, the current working
group has selected the most adequate data for OEL derivation.
Figure 1. Different CNTs. Left: Transmission electron microscope (TEM) image of
fiber-like MWCNTs (Poulsen et al. 2015b). Bar size: 1 µm. Middle: TEM image of
flexible MWCNTs (Poulsen et al. 2015b). Bar size: 1 µm. Right: TEM image of
SWCNTs (Balarak et al. 2016).
12
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
CNTs can be synthesized though different processes and the method chosen affect the
level of impurities and structural defects. In general, most synthesis processes take place
in vacuum, on a catalyst (often a metal such as Fe, Ni or Co), with controlled process
gasses and controlled temperature (Ismail et al. 2018). The most commonly used method
for commercially available CNTs is chemical vapor deposition (CVD). In this process, the
type of CNT, its structure and its size can be controlled by the size and type of catalyst,
the choice of gas and the temperature. This means that physical dimensions are well
controlled although it may result in many structural defects on the CNTs, especially
MWCNTs. Another commonly used method for large-scale production is arc discharge,
which is a fairly easy and inexpensive method. The disadvantage to this method is that
carbon black, fullerenes and soot are generated as byproducts and that the raw CNTs
formed often contain a lot of metal catalyst residues. More precise, but also more
expensive, CNT synthesis processes, as laser ablation, also exist.
The most common structural defects introduced during the synthesis process are missing
carbon atoms or replacement of hexagons by pentagons or heptagons. Such defects may
increase the curvature of the CNTs due to elongation or compression of one side of the
CNT (Zhang and Li 2006). It is also possible that structural defects could render the
graphene sheets and thereby the CNTs more susceptible to biological degradation,
which would change their toxic potential. Equally, the presence of bioavailable metal
impurities from catalysts on the surface of the CNT could have influence their toxic
potential. Some metals, especially iron, are known to induce reactive oxygen species
(ROS) (Knaapen et al. 2004). ROS may induce damage to cellular components such as
DNA, lipids and proteins, thereby causing genotoxicity.
Pure, graphitized CNTs are very hydrophobic in nature. Functionalization of CNTs is
therefore an important tool for increasing their solubility in aqueous solutions or their
chemical binding in solid composites. This is also important for the potential use of
CNTs in biological applications. By changing their polarity, the dispersion and the
biological interactions in the lung milieu, as well as the fate of the CNTs, may change
dramatically. This could ultimately change the toxic potential of the CNTs. Several
studies have reported altered toxicity of functionalized CNTs compared to pristine CNTs
(Hamilton, Jr. et al. 2013;Jain et al. 2011;Poulsen et al. 2016;Sager et al. 2014;Sayes et al.
2006).
The overall structure and composition of CNTs facilitate excellent electric and thermal
conductivity, high tensile strength, and good chemical stability (Dresselhaus et al. 2004).
Due to these abilities, CNTs are desirable for use in a variety of products, including
composite materials, electronics, plastics and rubbers, coatings, insulation and in
biomedical applications (De Volder et al. 2013;Jensen et al. 2015). As an example, the
high-aspect ratio and high tensile strength of CNTs makes them ideal for low weight
materials, e.g. sports equipment and wind mill wings. Due to their many possible
applications, CNTs are already produced and utilized at commercial scale, and they are
available worldwide. The global market for CNT products is expected to grow from an
estimated USD 3.43 Billion in 2016 to USD 8.70 billion in 2022 (Markets and Markets
2017). Thus with increased production, the potential exposure risks for both workers and
consumers have also increased.
13
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
To our knowledge, there are no legally enforced occupational exposure limits (OEL) for
CNTs. In Denmark, the approximate OEL is the current for bulk carbon black, which has
the same chemical composition as CNTs. The limit is 3.5 mg/m
3
and is regulated by the
Danish Working Environment Authority. The aim of the present report is to investigate
if the present knowledge allows for a suggestion of a health-based, OEL for CNTs. This
document will therefore review the relevant literature on the adverse effects of CNTs. As
suggested in the guidelines from REACH (ECHA 2012), the risk assessment
methodology in this report will be divided into threshold or non-threshold effects. The
threshold effect approach relies on the assumption that the organism can withstand a
certain dose before adverse effects occur, whereas non-threshold effects assume that any
exposure to the substance can result in adverse effects. For an OEL based on threshold
effects, the following traditional approach is utilized (ECHA 2012): 1) identification of
critical effect, 2) identification of the NOAEC, 3) calculation of OEL using assessment
factors adjusting for inter and intra species differences. For non-threshold effects, the
current working group will use two approaches. The first method, used by Kasai et al.
2016 and Erdely et al. 2013, uses the measured lung burden in rats exposed by inhalation
and the alveolar surface area of rats and humans to estimate the human equivalent lung
burden. The second method, suggested by (ECHA 2012;SCHER/SCCP/SCENIHR 2009),
uses air concentrations directly. Conclusively, the calculated OELs will be compared and
lastly, a recommended OEL for CNT exposure will be proposed.
14
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
H
UMAN STUDIES
Exposure
CNTs are synthesized as powders and inhalation is therefore considered the main route
of exposure in humans, although dermal exposure is also possible. Exposure though
inhalation may occur during the entire CNT lifecycle: Manufacturing, storage,
transportation, product application, and end-of-life processes. However, in their final
applications CNTs rarely exist as free fibers. When bound to a surface or suspended in
either liquids or solids, the individual CNTs are surrounded and more or less bound in
the matrix. It has been reported that this diminishes their toxic potential substantially
(Saber et al. 2016). Therefore the greatest risk of human exposure is in the working
environment, especially during production and handling of large quantities of free
CNTs. The nano-sized properties of CNTs are important for scenarios involving human
exposure in the occupational settings:
•
•
•
More individual CNT per mass unit compared to larger fibers.
Larger surface area per mass unit compared to larger fibers.
More dusty and therefore stays in the air longer compared to larger fibers.
Measurement of personal exposure levels to CNTs in occupational settings has been
reported in several studies (Table 1). Whereas the earlier exposure assessment studies
measured total inhaled mass or total gravimetric mass in the personal breathing zone
(Han et al. 2008;Lee et al. 2010;Maynard et al. 2004;Methner et al. 2010), the more recent
studies measured elemental carbon (EC) concentrations in personal breathing zone
samples (Birch et al. 2011;Dahm et al. 2012;Dahm et al. 2015;Lee et al. 2015;Methner et al.
2012;Ono-Ogasawara et al. 2015;Shvedova et al. 2016;Takaya et al. 2012;Erdely et al.
2013;Hedmer et al. 2014), which is more specific for carbon-based particles, including
CNTs. As a possible consequence of this, the older studies tended to report higher
exposure levels compared to newer studies. In general, the newer occupational exposure
assessment studies found personal breathing zone concentration levels ranging from
non-detectable to ca. 80 µg/m
3
. The differences in personal breathing zone concentration
levels are primarily attributed to different worker exposure scenarios. These studies
demonstrate that human exposures to CNTs occur in occupational settings.
15
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
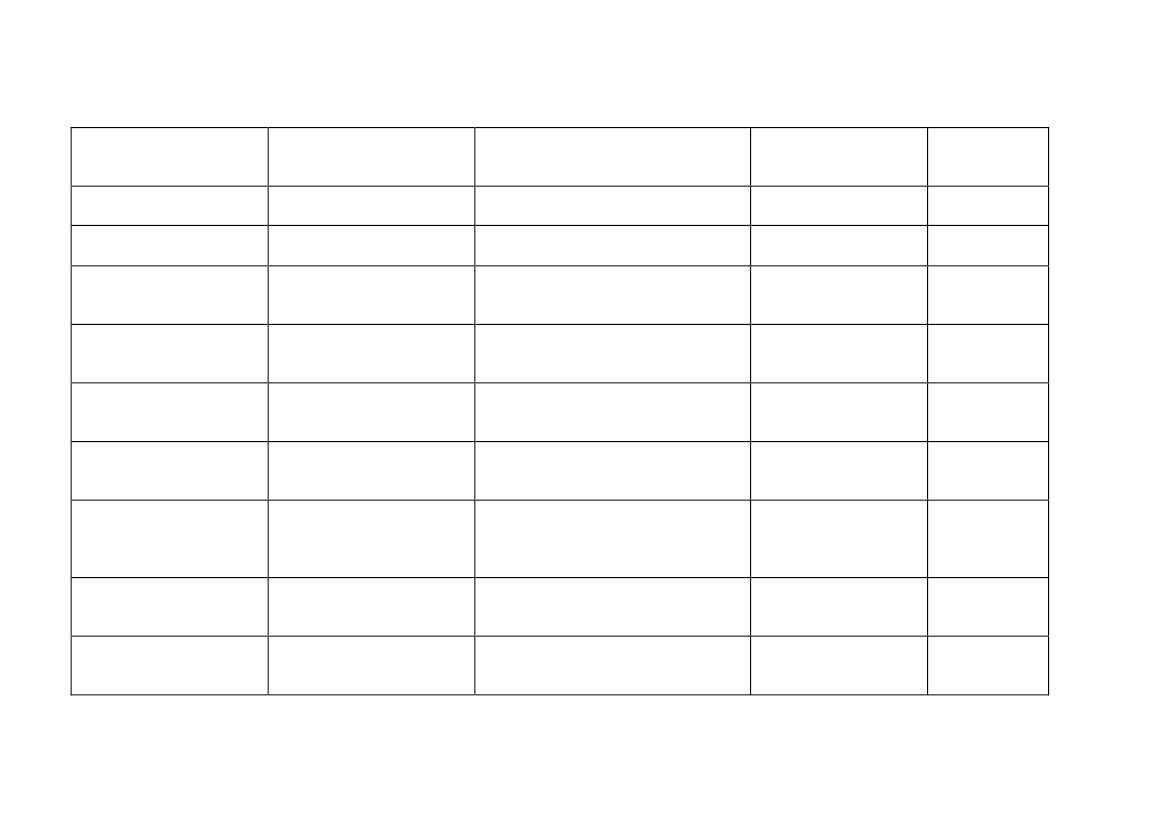
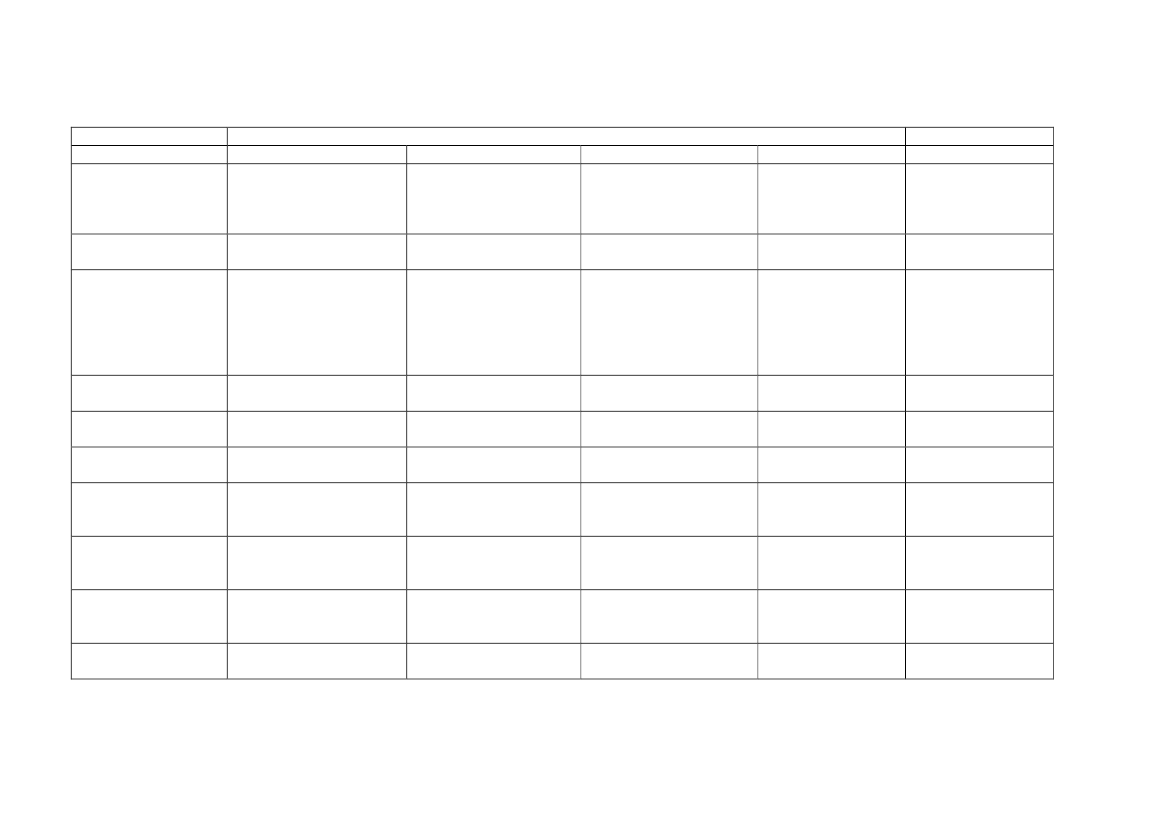
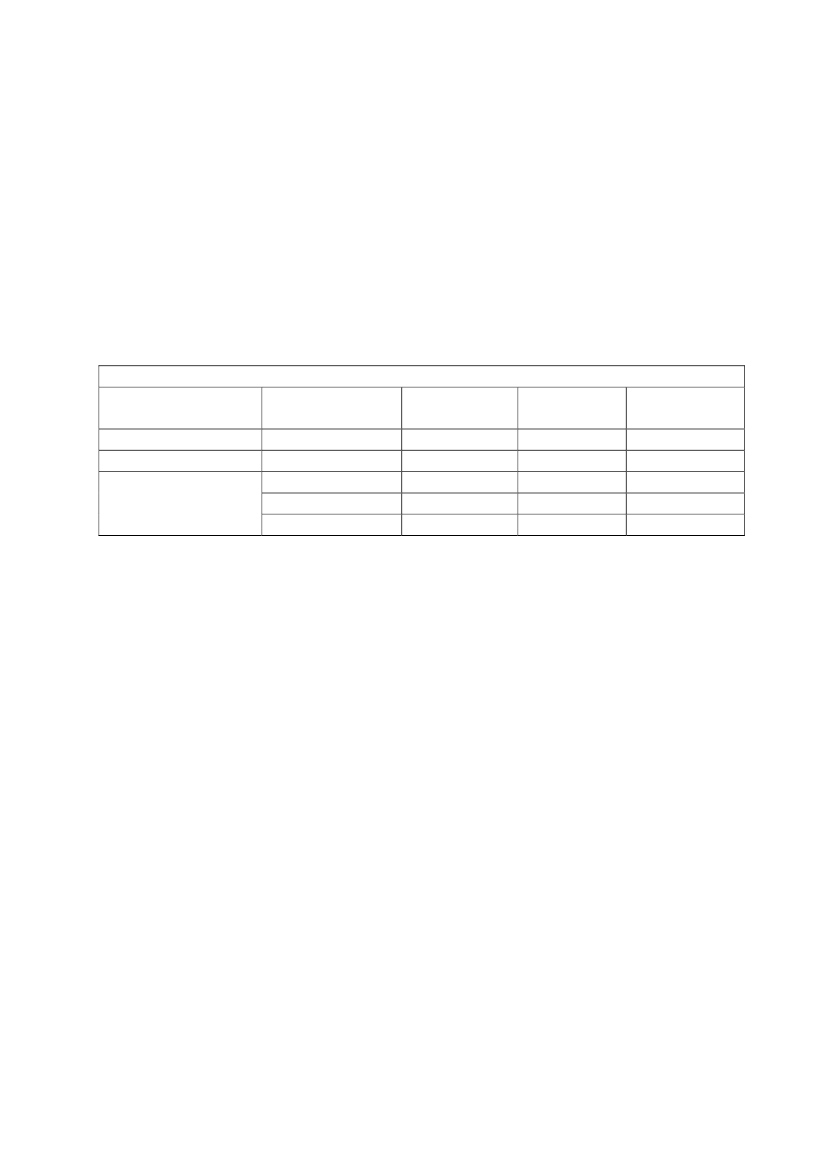
Table 1. Measured personal occupational exposure levels of CNTs.
Type of samples collected
Estimated inhalable mass
Total gravimetric mass
Total gravimetric mass
Work place
Four facilities producing
SWCNTs
MWCNT research facility
Work process
HiPCO or laser ablation production
Handling of MWCNTs: Blending,
weighing, spraying, miling, etc..
Production and handling of MWCNTs
Personal breathing
zone mass
concentrations (μg/m
3
)
0.7 - 53
N.D. - 331.7
7.8 - 320.8
Reference
Maynard et al.
2004
Han et al. 2008
Lee et al. 2010
Two research institutes, two
labs and three industrial
facilities
Total carbon-inhalable size
Five research and
Handling of CNTs: Weighing, mixing,
fraction
development labs, and one
wet sawing, processing, drying, etc.
manufacturer
Elemental carbon-
A facility manufacturing
Carbon nanofiber handling
respirable size fraction
and processes vapor-grown
carbon nanofibers
Elemental carbon-
Five facilities producing
Production and handling of CNTs:
inhalable size fraction
CNTs and one developer of Weighing, mixing, sonicating, milling,
semiconductor
etc.
Elemental carbon-
Two facilities producing
Production and handling of CNTs and
inhalable size fraction
CNTs and two facilities
carbon nanofibers: Weighing,
producing carbon nanofibers
spraying, filtration, cleaning,
harvesting
Respirable elemental
Factory creating fabric from
Weaving
carbon mass
yarns covered with
concentrations
MWCNTs
Elemental carbon-
Eight facilities producing or
Production and handling of CNTs:
inhalable size fraction
using MWCNT
Weighing, mixing, sonicating, milling,
etc.
64-1094
Methner et al.
2010
Birch et al.
2011
Dahm et al.
2012
Methner et al.
2012
45 - 80
N.D. - 7.86
N.D. - 38
3.5 - 4.8
Takaya et al.
2012
Erdely et al.
2013
N.D - 79.6
16
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Elemental carbon-
respirable size fraction
Inhalable elemental carbon
mass concentrations
Respirable elemental
carbon mass
concentrations
Respirable elemental
carbon concentrations
Small-scale producer of
MWCNTs by arc discharge
A MWCNT manufacturing
company
Primary and secondary
manufacturers of CNT or
carbon nanofibers
A MWCNT manufacturing
facility
Production and handling of
MWCNTs: Cleaving, sieving,
cleaning, harvesting, grinding, etc.
Manufacturing of MWCNTs
<0.08 - 7.4
Hedmer et al.
2014
Lee et al. 2015
Dahm et al.
2015
Shvedova et al.
2016
5.5 - 9.3
0.02 - 2.94
Production and handling of CNTs:
Weighing, mixing, sonicating, milling,
etc.
Production and handling of
MWCNTs:
Harvesting,
disintegration, packaging, laboratory
handling, etc.
0.54 - 6.11
N.D: Not detected, i.e. below detection limit.
17
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Biomonitoring
Although carbon nanotubes have been known since the mid 50’s, they did not receive
much attention from the scientific community as a whole until Sumio Iijima described
MWCNTs in his Science article from 1991 (Iijima 1991). CNTs are therefore a relatively
new material, and large-scale productions have only started within the last decade.
Consequently, studies reporting toxicological effects after both occupational and non-
occupational human exposure are very scarce. At present date, only one study is
available in the literature.
Shvedova and colleagues investigated biomarkers in induced sputum and blood of
workers exposed to MWCNTs in a manufacturing facility in Tambov, Russia (Nanotech
Center Ltd.) (Shvedova et al. 2016;Fatkhutdinova et al. 2016). They recruited 8 workers
exposed to MWCNTs (as having direct contact with MWCNT aerosol for at least 6
months) and 7 non-exposed controls from the same facility. Further, exposure
assessment of the personal breathing zone at different tasks in the facility was also
conducted. The 8-h, TWA elemental carbon concentrations in respirable size fractions
were measured at different workstations and were in the range of 0.7–2.8 µg/m
3
(Fatkhutdinova et al. 2016). The control group, who did not handle MWCNTs, was not
exposed to MWCNTs. Exposed workers had more than 2-fold increased serum levels of
IL1B, TNF, IL4 and IL10 and increased sputum levels of Il1B, TNF, IL6, IL4, IL5, IL8 all
indicative of systemic inflammation. Acute phase response proteins C-reactive protein
(CRP) and serum amyloid A (SAA) were not assessed. No no-observed-adverse-effect
concentration (NOAEC) or lowest-observed-adverse-effect concentration (LOAEC) was
calculated, but the authors of the current report note that the exposure levels were
relatively low at 0.7–2.8 µg/m
3
, indicating that even this low level was sufficient to
induce markers for systemic inflammation in humans.
18
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
T
OXICOKINETICS
The behavior and distribution of CNTs after exposure is of great importance for how and
where they affect the organism. The main entry way, which in the case of occupational
exposure is the lung and to some extent the skin, will undoubtedly receive the greatest
load and experience the greatest changes. However, extra-pulmonary alterations have
previously been reported after pulmonary exposure to CNTs (Poulsen et al.
2015a;Poulsen et al. 2017;Kim et al. 2015). Translocation of CNTs is a likely explanation
for these distal changes, although it is possible that secondary exposure through
inflammation plays a significant role. In order to understand potential target effects, it is
important to identify the tissues where CNTs accumulate.
Umeda and colleagues evaluated deposition pattern of MWCNTs MWNT-7 (D: 88±5 nm,
L: 5.0±4.5 µm) after inhalation exposure in F344 rats of both sexes (doses 0.2, 1 or 5
mg/m
3
MWCNT, 6 h/day, 5 days/week for 2 weeks)(Umeda et al. 2013). MWCNT
deposition was observed in the entire lung (bronchiolar space, alveolar space, alveolar
walls) and in the nasal cavity immediately after exposure. The MWCNTs were primarily
detected within alveolar macrophages with, a few free MWCNT fibers found in the
bronchi and alveolar space. The quantity of MWCNTs was higher in the rats exposed to
5 mg/m
3
MWCNTs compared to the lower doses. Deposited CNTs have been reported to
reach the sub-pleural region after pulmonary exposure in rodents (Ryman-Rasmussen et
al. 2009;Mercer et al. 2010). Inhaled MWCNTs (L:0.5–40 µm, D:10–50 nm, 1 or 30 mg/m
3
for 6 h) translocated rapidly to the sub-pleura region and were detected until the end of
the experiment 14 weeks post-exposure. The authors suggested that macrophages
facilitated the transport by engulfing the MWCNTs (Ryman-Rasmussen et al. 2009). In
concordance with this, Mercer et al. showed that 0.6% of the pulmonary deposited dose
had translocated to the sub-pleural region one day after pharyngeal aspiration of the
MWCNT MWNT-7 (L:3.9 µm, D: 49 nm)(Mercer et al. 2010). The translocation to the sub-
pleural region may suggest a similar mode of action as asbestos.
(Mercer et al. 2013) exposed male mice to MWCNTs MWNT-7 (aerodynamic diameter of
1.3 µm) by inhalation (5 mg/m
3
MWCNT aerosol for 5 hours/day for 12 days, 4
times/week for 3 weeks, estimated lung burden of 28.1 µg/lung). At 1 day and 336 days
after the exposure period they assessed the biodistribution of MWCNTs by darkfield
microscopy. They estimated that 7.3% of the lung burden measured at post-exposure day
1 was cleared from the lung at post-exposure day 336. The vast majority of this had
translocated to the lymph nodes, however 0.03% of the initial MWCNT lung burden was
found in the liver and lesser percentages was found in the kidney, heart, brain and
diaphragm.
Lung clearance and translocation to liver and spleen was assessed by (Czarny et al.
2014), who exposed female Balb/c mice to 20 µg
14
C skeleton-labelled MWCNTs by
pharyngeal aspiration. The MWCNTs had an average diameter of 41 nm, were 3.9 µm
long and appeared as straight single fibers in EM pictures. The limit of detection of the
radioactive labelling was determined to be in the order of 0.2 pg or 22 CNT fibers. Half
the dosed MWCNTs were cleared from the lung within 1 day. Of the remaining 10 µg,
10% was detected in the lung tissue 3 and 12 months post-exposure. At 12 months post-
exposure, 0.75% was found in liver and 0.20% was found in spleen. MWCNT
19
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
translocation to liver and spleen was confirmed with EM. Thus, this study shows that
rigid MWCNTs was cleared from the lung and to some extent translocated to the liver
and spleen. 10% of the dosed MWCNTs remained in the lung and no further clearance
was detected the period between 3 and 12 months post-exposure. Based on the data
provided in the paper, lung clearance of the alveolar deposited dose is estimated to
occur with a half-life of 30 day.
In a two-year inhalation study, rats were exposed MWNT-7 at doses 0.02, 0.2 and 2
mg/m
3
(Kasai et al. 2016). The MWCNTs had diameters of 92.9-98.2 nm and mean lengths
of 5.8-5.9 um, and were mainly found as single fibers. Lung burden increased linearly
over time in a dose-dependent manner. Deposited dose was estimated to be 1.5-2.7% of
the inhaled dose. Deposited dose per lung differed between sexes, but no difference was
observed when normalized to lung weight. In addition to the lung, the MWCNTs were
observed as single or aggregated fibers in nasal cavity, larynx, trachea, lungs, lymph
nodes, spleen, liver, kidneys, olfactory bulb, and brain. In the kidney, olfactory bulb and
brain, the MWCNTs were only observed as single fibers.
Pauluhn performed a 13-week inhalation study in rats using Baytubes, which were 10
nm in diameter and 200-300 nm long (Pauluhn 2010b). The MWCNTs appeared curved
on EM pictures. The aerosol consisted mainly of CNT agglomerates. Pulmonary
clearance in the rats was estimated by assessing Co content in the lung. Half-lives for
clearance were 150-375 days. The highest clearance rate was observed for the highest air
concentration. The current working group considers the highest dose to give the best
estimate, as the Co content was close to the limit of detection.
Taken together, the data suggest that agglomerated CNTs are cleared away from the
lung slower than CNTs that are dispersed as single fibers. In agreement with this,
Pauluhn and Rosenbruch have shown that CNT clearance following inhalation exposure
in rats occurs faster with well-dispersed Baytubes CNTs compared to aggregates of the
same CNT (Pauluhn and Rosenbruch 2015). Half-lives for pulmonary clearance were
estimated to be 87 and 46 days for aggregated and dispersed CNTs, respectively.
Translocation can in principle occur either by translocation from lung to blood or by
secondary uptake via the GI tract following pulmonary clearance by mucociliary
transport. However, no uptake was detected following oral dosing of the radioactively
labelled MWCNTs suggesting that translocation occurred from lung to systemic
circulation (Czarny et al. 2014).
20
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
A
NIMAL STUDIES
Rodent versus human response
As almost no human data on toxicity and epidemiological studies is available, inhalation
studies in mice and rats are used to assess potential human hazard.
One human study reported MWCNT-induced systemic inflammation following
exposure to relatively low air concentrations of MWCNT (Shvedova et al.
2016;Fatkhutdinova et al. 2016). This finding is in overall agreement with strong
inflammatory potential of several different CNTs in sub-chronic inhalation studies in
rats (Pauluhn 2010b;Ma-Hock et al. 2009;Kasai et al. 2016;Kasai et al. 2015), although no
quantitative comparison of the responses has been performed.
Rats are the preferred animal model in particle toxicology and more sensitive than mice
to particle-induced lung cancer and fibrosis. However, rats do not express the acute
phase proteins serum amyloid a isoforms Saa1, Saa2 and Saa3, which are expressed by
humans and mice (Saa3 in mice only)(Cray et al. 2009). Serum amyloid is causally related
to plaque formation (Thompson et al. 2018). Since rats do not express SAA, a key acute
phase protein, they may be less well suited as model of human hazard assessment of
atherosclerotic effects. In this case, mice would be a more accurate model animal. Like
mice, humans also express SAA in lung tissue (Calero et al. 2014). Particle-induced acute
phase response in terms of increased SAA and CRP levels in blood was recently shown
in human volunteers following inhalation of ZnO nanoparticles (Monse et al. 2018).
Intratracheal instillation versus inhalation
Inhalation studies are the gold standard of toxicity testing, as this exposure route is the
closest surrogate to human exposure. For practical reasons, pulmonary deposition by
intratracheal instillation is widely used in screening studies (Bourdon et al. 2012b;Husain
et al. 2013;Poulsen et al. 2015b;Saber et al. 2012b;Saber et al. 2012a) and has been
proposed as an alternative to inhalation exposure. This exposure method ensures that
the same dose is delivered to the lung for all nanomaterial exposures, demands less
material and is more user-friendly. Intratracheal installation has previously been shown
to give widespread distribution of particles throughout the lung (Mikkelsen et al. 2011),
also for MWCNT (Poulsen et al. 2016).
A number of studies have compared the toxicological response following inhalation and
instillation of nanomaterials. Two studies have compared the global transcriptional
profiles as a means to investigate the pulmonary biological response after inhalation
compared to instilled or aspirated nanomaterials. Inhalation and intratracheal instillation
of a surface modified TiO
2
NP resulted in similar transcriptional changes, with the acute
phase response and inflammation as the most important pulmonary responses to inhaled
and instilled TiO
2
(Halappanavar et al. 2011;Husain et al. 2013). Similarly, (Kinaret et al.
2017) compared the global transcriptomic profiles of lung tissue from mice exposed to a
straight and long MWCNT by inhalation or aspiration. The authors concluded that the
perturbed pathways were very overlapping, suggesting that the transcriptiomic
response to MWCNT exposure was very similar for inhaled and pulmonary dosed
MWCNTs.
21
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Other studies compared levels of pulmonary inflammation, measured as neutrophil
influx, after exposure by inhalation or intratracheal instillation in rodents. Two studies
using MWCNT reported that both methods resulted in pulmonary inflammation, with
inhalation being more potent at inducing inflammation (Morimoto et al. 2012b;Porter et
al. 2013). Baisch et al. reported that instillation of a high dose of TiO
2
NPs induced
greater inflammation compared to low dose rate delivery through inhalation, even
though the same pulmonary deposited dose were delivered. The authors concluded that
intratracheal instillation is useful for quantitative ranking of NP hazards, but not for
quantitative risk assessment (Baisch et al. 2014).
Selection of studies and endpoints
In the present report inhalation studies will be prioritized. For the description of
toxicological endpoints and mechanism of toxicity, studies using pulmonary deposition
as intratracheal instillation will be included where no quality inhalation studies are
available. Risk assessments, however, are solely conducted based on inhalation studies.
Hazard endpoints were evaluated based on reported adverse effects of CNT exposure in
reports and in the scientific literature. Previous assessments on CNTs have mainly
focused on inflammation as critical effect. However, these evaluations were conducted
prior to the pivotal, long term inhalation study in rats investigating the carcinogenic
potential of the long, thick MWCNT called MWNT-7 (Kasai et al. 2016) and the IARC
classification of MWNT-7 as possibly carcinogenic (2B) (Grosse et al. 2014). This report
will therefore include both endpoints. In addition, cancer and cardiovascular disease
have been identified as two of the main mortality causing diseases in the world, with a
combined estimate of approximately 26 million annual deaths worldwide (World Health
Organization 2018;Cancer Risks UK 2018). Both diseases are potentially initiated by
inflammation, as described in
Mechanism of toxicity.
In conclusion, the critical endpoints
were chosen based on literature review, mechanistic understanding and general
importance in regards to worldwide mortality rates.
Pulmonary inflammation
Pulmonary inflammation, and inflammatory-related changes, is the most commonly
reported adverse effect of CNT exposure. However, the inflammogenic potential of
CNTs varies and is largely dependent on deposited dose and their physico-chemical
properties. The influx of neutrophilic cells into the BAL fluid is a commonly used and
reliable marker of pulmonary inflammation (Ma-Hock et al. 2009;Morimoto et al.
2012a;Erdely et al. 2009). Studies have shown that neutrophil influx correlates with
increases in both pro-inflammatory cytokine and acute phase response mRNA, and
protein levels (Bourdon et al. 2012b;Bourdon et al. 2012a;Bourdon et al. 2013;Husain et
al. 2013;Jackson et al. 2013;Poulsen et al. 2013;Poulsen et al. 2015b). In this report, the
current working group therefore chose pulmonary neutrophil influx in BAL fluid as the
marker of pulmonary inflammation.
22
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Due to the large number of studies reporting pulmonary inflammation after exposure to
CNTs, the current working group chose to highlight quality chronic and sub-chronic
inhalation studies (Table 2).
Chronic inhalation studies
One inhalation study was identified (Kasai et al. 2016). Here, rats were exposed to
MWCNTs MWNT-7 6h/day, 5 days/week for 2 years (D: 92.9-98.2 nm, L: 5.8-5.9 µm).
Statistically significantly increased neutrophil influx was observed at 2 mg/m
3
, but not at
0.2 or 0.02 mg/m
3
(Kasai et al. 2016). LOAEC for MWNT-7 in this study was therefore 2
mg/m
3
and NOAEC 0.2 mg/m
3
. The LOAEC and NOAEC levels for neutrophil influx
were lower in this chronic study, compared to the levels in the sub-chronic study of
5 mg/m
3
and 1 mg/m
3
, respectively, from the same authors (Kasai et al. 2015). This
indicates that the severity of pulmonary inflammation increases with exposure time and
thereby with increasing deposited dose.
Sub-chronic inhalation studies
Four different 13-week inhalation studies performed according to the OECD guidelines
have been published using 4 different MWCNTs (Ma-Hock et al. 2009;Pauluhn
2010b;Pothmann et al. 2015;Kasai et al. 2015). In all studies, rats were exposed 6h/day, 5
days/week for 13 weeks (Table 2).
Ma-Hock and colleagues exposed Wistar rats to aerosols of a short and thin MWCNT
type (D: 5-15 nm, L: 0.1-10 µm) at doses 0.1, 0.5, 2.5 mg/m
3
(Ma-Hock et al. 2009). The
authors reported no histopathological changes in any organ, except the lung, after the 13
weeks of exposure. Lung findings were dose-dependent and included increased lung
weights, pronounced multifocal granulomatous inflammation, diffuse histiocytic and
neutrophilic inflammation, and intra-alveolar lipoproteinosis at doses at 0.5 and 2.5
mg/m
3
. The investigators did not observe pulmonary fibrotic effects in the exposed rats.
At the lowest exposure level, 0.1 mg/m
3
, the authors reported minimal granulomatous-
type inflammation in the lungs and lung-associated lymph nodes, which were
considered sub-clinical and unlikely to be associated with functional effects. Based on
the observations reported by the authors, a NOAEC of 0.1 mg/m
3
and LOAEC of 0.5
mg/m
3
were established for this MWCNT type.
In the second sub-chronic inhalation study, Pauluhn exposed Wistar rats in nose-only
chambers to a short and thin MWCNT type (D: 10 nm, L: 0.2-0.3 µm) at doses 0.1, 0.4, 1.5,
and 6 mg/m
3
(Pauluhn 2010b). Pulmonary effects were examined up to 6 months post
exposure. Sustained pulmonary inflammation, in terms of increased neutrophil influx in
BAL fluid, was observed at doses 0.4 mg/m
3
and up. At 0.4 mg/m
3
and above, exposure-
related lesions in the upper and the lower respiratory tracts were revealed by
histopathology. Focally increased interstitial collagen staining, indicative of fibrosis, was
observed at doses 1.5, and 6 mg/m
3
, with borderline effects at dose 0.4 mg/m
3
. All
endpoints increased in intensity from exposure weeks 8 to 13, followed by a time-
dependent decrease in severity for all exposure groups. Based on the observations of the
study, a NOAEC of 0.1 mg/m
3
and LOAEC of 0.4 mg/m
3
were established for this
MWCNT type.
23
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Pothmann and colleagues exposed Wistar rats to a short and thin MWCNT type (D: 12.1
nm, L: 1.07 ± 1.1 µm) by nose-only exposure at doses 0.05, 0.25 and 5.0 mg/m
3
(Pothmann
et al. 2015). The animals were euthanized 1 or 90 days after the last exposure.
Significantly increased neutrophil levels were observed after exposure to the highest
dose (5.0 mg/m
3
) immediately after last exposure and at doses 0.25 and 5.0 mg/m
3
90
days after exposure. These increases were accompanied by increased levels of cytokines
IL-1β, IL-5, TNF-α, and IL-1
α.
Histopathological examination revealed focal/multifocal
collagen depositions after exposure to 5.0 mg/m
3
. Based on the observations of the study,
a NOAEC of 0.05 mg/m
3
and LOAEC of 0.25 mg/m
3
were established for this MWCNT
type.
In the last sub-chronic inhalation study, Kasai and colleagues exposed F344 rats by
whole-body inhalation to a long and thick MWCNT type (MWNT-7)(D: 94.1-98.0 nm, L:
5.53-6.19 µm) at doses 0, 0.2, 1 and 5 mg/m
3
(Kasai et al. 2015). In contrast to the previous
sub-chronic inhalation studies, the animals were exposed to aerosolized single fibers,
instead of agglomerates. The authors reported increased neutrophil influx in BAL fluid
at dose 1 mg/m
3
and granulomatous changes in the lung at dose 0.2 mg/m
3
and up.
Histopathological examination revealed focal fibrosis of the alveolar wall at dose 1
mg/m
3
and up. Based on the observations of this study, a NOAEC of 0.2 mg/m
3
and
LOAEC of 1 mg/m
3
for inflammation were established for this MWCNT type. However,
lactate dehydrogenase and alkaline phosphatase activities, and total protein levels were
all increased after exposure to 0.2 mg/m
3
MWCNTs.
Three of the four MWCNTs were thin (5-12 nm in diameter) and were aerosolized as
dense agglomerates. The BET surface area of these MWCNTs was approximately 10
times larger than the BET surface area of the fourth MWCNT, MWNT-7. The observed
NOAEC based on neutrophil influx was 0.05-0.1 mg/m
3
for the thin MWCNTs and 1
mg/m
3
for the thick MWNT-7, and thus appeared proportional to the BET.
Conclusion
In general, the chronic and sub-chronic inhalation studies identified NOAECs ranging
from 0.05 mg/m
3
to 1 mg/m
3
and LOAECs ranging from 0.25 mg/m
3
to 5 mg/m
3
(Table 2).
As dose-dependency was identified for inflammation and as it was possible to detect a
NOAEC, inflammation is considered a threshold effect.
24
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
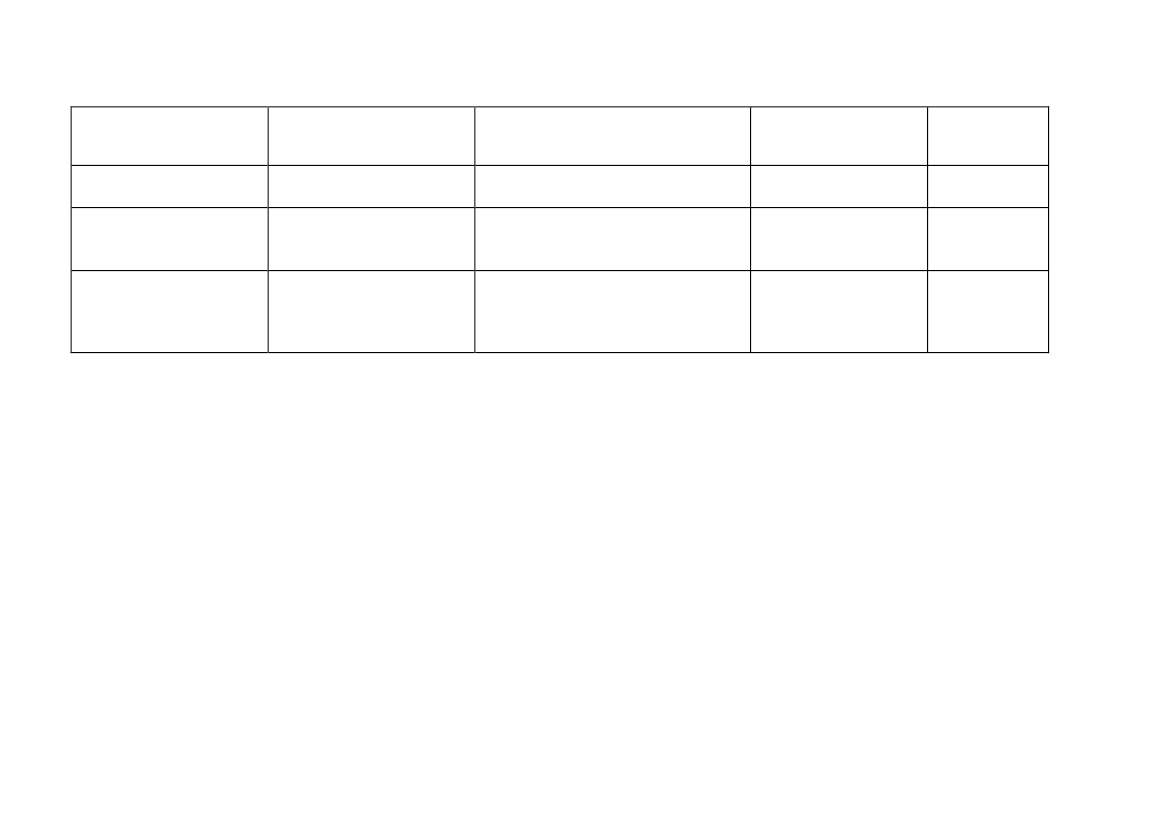
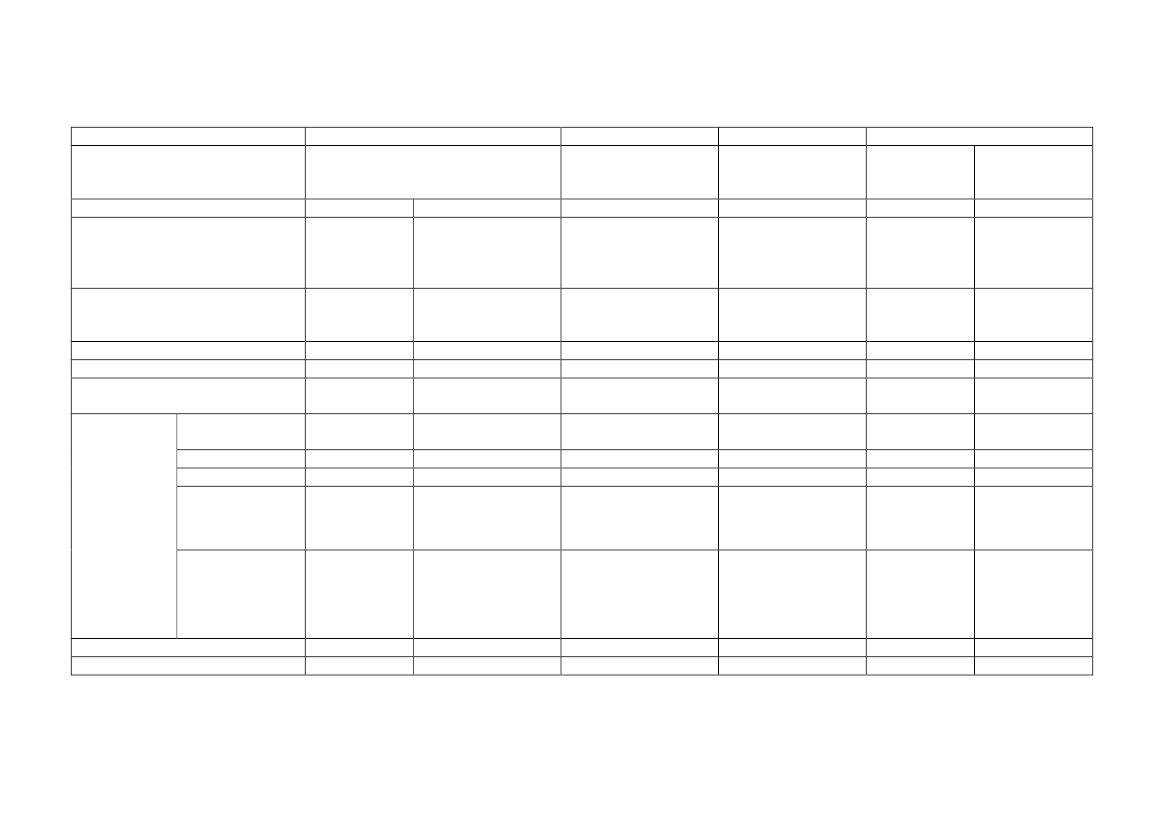
Table 2. Overview of sub-chronic and chronic inhalation studies in rats with inflammation as an endpoint
Sub-chronic studies
Ma-Hock et al. 2009
Pauluhn 2010b
Pothmann et al. 2015
Kasai et al. 2015
Rat strain
Male and female Wistar
Male and female Wistar
Male and female rats,
Male and female
rats (N=10/group)
rats (N=6-10 per group)
RccHan©: WIST(SPF)
F344 rats
(N=10/group)
(N=10/group)
MWCNT
MWCNT Dimensions
Nanocyl NC 7000
D: 5-15 nm
L: 0.1-10 µm
BET: 250-300 m
2
/g
Aerosolized as respirable
agglomerates
0.1, 0.5, 2.5 mg/m
3
6h/day, 5 days/week for
13 weeks
At the end of exposure
0.1 mg/m
3
0.5 mg/m
3
Baytubes
D: 10 nm
L: 0.2-0.3 µm
BET: 253 m
2
/g
Aerosolized as respirable
agglomerates
0.1, 0.4, 1.5, 6 mg/m
3
6h/day, 5 days/week for
13 weeks
1 day, 28 days, 13 w, 26
w
0.1 mg/m
3
0.4 mg/m
3
0.1 mg/m
3
0.4 mg/m
3
(females not
assessed)
Graphistrength© C100
(NM-402)
D: 12.1 nm
L: 1.07 ± 1.1
μm
BET: 225.6 m
2
/g
Aerosolized as respirable
agglomerates
0.05, 0.25 and 5.0 mg/m
3
6h/day, 5 days/week for
13 weeks
1 and 90 days
0.05 mg/m
3
0.25 mg/m
3
0.25 mg/m
3
5 mg/m
3
MWNT-7
D: 94.1-98.0 nm
L: 5.53-6.19 µm
BET: 24-28 m
2
/g
Aerosolized as single
fibers
0.2, 1, 5 mg/m
3
6h/day, 5 days/week
for 13 weeks
After overnight
fastening
1 mg/m
3
5 mg/m
3
0.2 mg/m
3
based on
LDH, ALP and total
protein in BALF
1 mg/m
3
Chronic study
Kasai et al. 2016
Male and female
F344/DuCrlCrlj rats
(N=50/group)
MWNT-7
D: 92.9-98.2 nm
L: 5.8-5.9 µm
BET: 24-28 m
2
/g
Aerosolized as single
fibers
0, 0.02, 0.2, and 2
mg/m
3
6h/day, 5 days/week
for 104 weeks
At the end of
exposure
0.2 mg/m
3
2 mg/m
3
0.02 mg/m
3
0.2 mg/m
3
Air concentrations
(mg/m
3
)
Exposure setup
Post exposure time
points
NOAEC neutrophil
influx 1 day post-
exposure
LOAEC neutrophil
influx
1 day post-exposure
NOAEC fibrosis
1 day post-exposure
LOAEC fibrosis
1 day post-exposure
No fibrosis reported
No fibrosis reported
25
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Genotoxicity and cancer
Genotoxicity and cancer are well studied, possible adverse effects of exposure to CNTs.
Genotoxicity often occurs relative rapidly after exposure, whereas cancer is a more
complex pathological endpoint that requires longer time to develop. In this report the
current working group therefore chose to differentiate between genotoxicity in shorter-
term studies and cancer in long-term studies.
Genotoxicity
The genotoxic potential, and thus the ability to induce cancerous changes, is likely
depended on the physico-chemical properties of the CNTs. Indeed, studies have
highlighted differences in CNTs’ ability to induce genotoxicity. Pulmonary exposure by
intratracheal instillation to 10 different MWCNTs (diameters 13-32 nm) in mice (doses: 6-
54 µg) increased DNA strand break levels in both BAL fluid and lung tissue for some of
the MWCNTs (Poulsen et al. 2016). No dose-response was observed. Using linear
regression analysis with independent physico-chemical parameters, the authors
identified diameter thickness as a possible predictor for DNA strand break levels in BAL
cells and lung tissue, even for these relatively thin MWCNTs. In concordance with this,
another study reported increased DNA strand breaks following pulmonary dosing of the
long and straight MWCNT MWNT-7 (1–200 µg/mouse by pharyngeal aspiration and
8.2–10.8 mg/m
3
for 4 days, 4 h/day by inhalation), but not a thin and entangled CNT
(diameter 8-15 nm) (Catalan et al. 2016). This could indicate that needle-like CNTs are
more genotoxic than thinner and often more entangled CNTs.
However, pulmonary exposure by intratracheal instillation of straight, thick and long
MWCNTs (D: 67 nm, L: 4 µm), and thin and short MWCNTs (D: 10 nm, L: 1.5 µm) in
mice both increased DNA strand break levels in lung tissue across doses (18-162 µg) and
time points (1-28 days) (Poulsen et al. 2015b). Similarly, two sub-chronic inhalation
studies in rats using thin CNTs (D: 10-15 nm and 12.1 nm, respectively) at comparable
doses reported opposing genotoxic potential. The first study showed increased levels of
DNA strand breaks in lung tissue from male and female rats following 28 days
inhalation (0.17-0.96 mg/m
3
, 6 h/day, 5 days per week) (Kim et al. 2014), whereas the
other reported no effect on DNA strand break levels, oxidative DNA damage or
micronuclei formation after 90 days inhalation (0.05-5.0 mg/m
3
, 6 h/day, 5 days per
week) (Pothmann et al. 2015). This indicates that other factors than diameter thickness
play a part in CNT-induced genotoxicity.
Cancer
The MWCNT MWNT-7 (D: 92.9-98.2 nm, L: 5.8-5.9 µm) was reported to cause peritoneal
mesotheliomas up to one year after non-pulmonary deposition in two studies (3-300 µg
in p53 heterozygous mice, or 0.5 or 5 mg MWCNT in rats twice with a 1-week interval,
respectively) (Takagi et al. 2012;Nagai et al. 2011). The same MWCNT also promoted
bronchioloalveolar adenoma and carcinoma in male mice after inhalation (5 mg/m
3
, 5
h/day, 5 days/week, 3 weeks) (Sargent et al. 2014). Based on these studies IARC classified
MWNT-7 as possibly carcinogenic (2B) (Grosse et al. 2014). In contrast, intraperitonal
injection of 2 or 20 mg short and thin (11 nm diameter, length ca 0.7 um) with or without
26
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
structural defects in rats did not induce abdominal tumors up to 2 years after exposure,
whereas the positive control crocidolite asbestos (2 mg) did (Muller et al. 2009).
After publication of the IARC evaluation, more studies investigating the carcinogenic
potential of CNTs were published. (Rittinghausen et al. 2014) showed that IP injection of
4 different long (>7.9 um) and relatively thick (diameter: 37-85 um) MWCNTs at dose
levels of 1 and 5x10
9
fibers induced malignant mesotheliomas in a dose-dependent
manner for each of the studied MWCNTs. As the MWCNTs were dosed as 1 or 5x10
9
fibers, the dosed mass of the MWCNTs differed significantly between the 4 MWCNTs.
(Suzui et al. 2016) exposed rats to 1 mg NIKKISO MWCNTs by trans-tracheal
intrapulmonary spraying into the lung. MWCNTs were fractionized by size by filtering
through a 25 µm pore size sieve. The mean lengths of the MWCNT fractions were:
Unfiltered: 4.2 +/- 2.9 µm, flow-through: 2.6 +/- 1.6 µm, and retained > 2.6 µm. The
physico-chemical properties of NIKKISO were very similar to MWNT-7, except that the
flow-through fraction was shorter. The rats were exposed to the MWCNTs fractions 8
times during a 2 week period with a total dose of 1 mg/rat. The groups consisted of 12-15
rats and they were followed for 109 weeks post-exposure. Lung tumors were observed in
the combined three groups (37%) with no statistically significant differences between
groups. Mesotheliomas were statistically significantly increased in the combined group,
and were observed in the unfiltered and flow-through fractions although not statistically
significant. No mesotheliomas were observed in the retained fraction. NIKKISO were
carcinogenic even though the fibers were relatively short compared to MWNT-7, and no
difference in lung cancer incidence was observed across the different fractions. This
indicates that the length of MWCNTs is of less importance for their ability to promote
cancer.
A pivotal 2 year inhalation study investigated pulmonary pathological changes after
exposure to MWCNTs MWNT-7. F344 male and female rats (N=50 per exposure group)
were exposed to MWNT-7 for 6 h/day, 5 days/week for 104 weeks at concentrations of 0,
0.02, 0.2, and 2 mg/m
3
(Kasai et al. 2016). Scanning electron microscope of MWNT-7
demonstrated that most MWCNTs were aerosolized as single straight fibers with a mean
length of 5.8-5.9 µm.
The incidences of bronchiolo-alveolar carcinomas, total carcinomas (bronchiolo-alveolar
carcinomas, adenosquamous carcinoma, adenocarcinoma and squamous cell carcinoma),
and total carcinomas and/or adenomas were significantly increased in males exposed to
0.2 and 2 mg/m
3
MWNT-7 and females exposed to 2 mg/m
3
MWNT-7 compared with
their respective control groups (Table 3)(Kasai et al. 2016). The incidence of malignant
mesothelioma was not increased.
27
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Table 3. Total incidence of adenoma and/or carcinoma in the lungs of rats in the two
year inhalation study by (Kasai et al. 2016).
MWNT-7
0 mg/m
3
0.02 mg/m
3
0.2 mg/m
3
2 mg/m
3
concentration
Female rats
3/50
2/50
4/50
11/50*
Male Rats
2/50
2/50
13/50**
16/50**
*) p<0.05 **) p<0.01 by Fischer’s exact test
The total deposited MWCNT dose was calculated based on the number of fibers
observed and was concentration dependent. At 2 mg/m
3
, deposited dose was
approximately 1.8 mg/lung and 1.2 mg/lung for male and female rats, respectively, and
at 0.2 mg/m
3
it was approximately 0.15 and 0.12 mg/lung for male and female rats,
respectively. The number of fibers per gram body weight that induced lung carcinoma
was calculated to be 3.92 × 10
6
MWNT-7 fibers/gram body weight in males and 42.5 × 10
6
MWNT-7 fibers/gram body weight in females (Kasai et al. 2016).
In addition to the cancerous effects, exposure to MWNT-7 MWCNTs also induced
increased lung weight, pulmonary hyperplasia, granuloma formation and focal fibrosis,
and increased levels of total protein, lactate dehydrogenase, and alkaline phosphatase in
the BAL fluid at doses 0.2, and 2 mg/m
3
(Kasai et al. 2016). The dose of 2 mg/m
3
also
induced focal fibrosis in the pleura.
Conclusion
One specific thick and long carbon nanotube, MWNT-7/Mitsui-7/XNRI-7, has been
classified as possibly carcinogenic by IARC. After publication of the IARC evaluation,
the same MWCNT has been shown to induce lung adenomas and carcinomas by
inhalation in male (at 0.2 and 2 mg/m
3
) and female rats (at 2 mg/m
3
). Pulmonary dosing
of 1 mg/rat NIKKISO MWCNTs, which were shorter than MWNT-7, but otherwise with
similar physico-chemical properties, caused lung cancer. Other thick (>37 nm) and long
(>7.9 µm) have been shown to cause cancer following IP injection, whereas IP injection of
high doses (2 and 20 mg) of a thin (diameter 11 nm) and short (0.7 um) MWCNT did not
cause cancer in a 2-year study in rats. It is therefore very probable that other thick and
straight MWCNTs are equally carcinogenic by inhalation. No firm conclusions can be
reached regarding length because of the large heterogenicity of the tested samples.
Dose-response relationships have been identified for MWCNT-induced carcinogenic
effects in several independent studies (Kasai et al. 2016;Rittinghausen et al. 2014;Takagi
et al. 2012). However, in the study by (Rittinghausen et al. 2014) several different
MWCNTs were compared, and the carcinogenic potential could not be explained by
mass or by the number of CNT fibers. In contrast to the cancer studies, no consistent
dose-response relationship has been observed for CNT-induced DNA strand breaks in
the comet assay (Poulsen et al. 2016;Catalan et al. 2016;Poulsen et al. 2015b). Due to the
lack of dose-response relationship and the severity of cancer as a disease, the current
working group has therefore decided to consider genotoxicity and cancer as non-
threshold effects, as this is the most conservative approach.
28
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Cardiovascular effects
The term cardiovascular effects cover pathological changes in the entire cardiovascular
system. Atherosclerosis is a central cardiovascular disease, which is manifested as
increased plaque deposition or build-up in the arteries. In the later stages this can lead to
various other cardiovascular diseases, including coronary artery disease and stroke. For
CNT-induced cardiovascular effects, the present working group will focus on studies
using intratracheal instillation as pulmonary exposure route, as these adverse effects
primarily have been investigated in such studies. Differentiation between studies
reporting primary changes, such as accelerated plaque progression, and studies
reporting changes related to/or leading to cardiovascular effects, such as the acute phase
response, will be conducted.
Accelerated plaque progression
The lipid profile of mice differs significantly from that of humans. In mice, rapid
clearance of hepatic low-density lipoproteins (LDL) results in low and rather stabile total
serum cholesterol levels, even after increased cholesterol intake and synthesis.
Atherosclerotic changes are therefore mainly investigated in ApoE -/- mice, which are
deficient in apolipoprotein E (apoE), a glycoprotein associated with all lipoproteins
except LDL. ApoE -/- mice develop spontaneous atherosclerosis as early as 3–4 months
of age when fed normal chow (Nakashima et al. 1994). This makes them suitable models
for investigating cardiovascular effects.
Several studies have reported CNT-induced accelerated plaque progression.
Intrapharyngeal instillation of 20 µg SWCNTs every other week for 8 weeks (total dose:
80 µg) in ApoE -/- mice fed either regular chow or high fat chow, revealed that SWCNT
induced accelerated plaque formation in mice fed on high fat chow (Li et al. 2007)
Similar increase was also observed in ApoE -/- mice exposed to SWCNT or DWCNT
once weekly for 10 weeks to 10 or 40 µg by pharyngeal aspiration (total dose 100 and 400
µg/mouse)(Suzuki et al. 2016). The authors reported accelerated plaque formation at the
high dose of 10 times 40 µg for both SWCNT and DWCNT. Two different short and thin
(D: 10-11 nm, L: 1.5 µm) MWCNT (NM-400 and NM-402) also induced accelerated
plaque formation in female ApoE -/- mice fed western diet after intratracheal instillation
once a week for 5 weeks to 25.6 µg/mouse (total dose 128 µg)(Cao et al. 2014).
In contrast, exposure to 4 or 40 µg of the thick and long MWCNT MWNT-7 (L: 3.86-5.7
µm, D: 49–74 nm) once a week for 10 weeks (total dose 40 or 400 µg) by intratracheal
instillation did not affect plaque formation in female ApoE -/- mice fed western diet
(Christophersen et al. 2016). The results indicate that accelerated plaque formation is
observed following pulmonary exposure to thin (SWCNT, DWCNT, NM-400 and NM-
402) but not thick (MWNT-7) MWCNT.
Acute phase response
The acute phase response is induced in humans in response to infection, infarction and
trauma, and it is defined by increases in acute phase response proteins with the most
predominant being C-reactive protein (CRP), Serum amyloid A (SAA), and fibrinogen.
During an acute phase response these proteins can increase thousand fold (Gabay and
29
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Kushner 1999). Elevated plasma levels of CRP and SAA have been reported as a risk
factor for CVD in humans (Johnson et al. 2004;Lowe 2001;Lowe 2001;Mezaki et al.
2003;Ridker et al. 2000). In mice, the SAA isoforms are the main acute phase response
proteins, while CRP is only moderately induced by inflammatory stimuli (Whitehead et
al. 1990;Pepys and Hirschfield 2003). SAA (SAA1-4) is a highly conserved family of
apolipoproteins associated with high density lipoproteins (HDL).
Several studies have reported changes in
Saa
expression or increased SAA protein levels
after pulmonary exposure to CNTs. Intratracheal instillation of both the thick MWCNT
MWNT-7 (D: 40–50 nm, L: 1– 4 µm) and two SWCNTs (D: 0.8–1.7nm, L: <1 µm) in female
C57BL/6 mice strongly increased
Saa3
mRNA levels in lung tissue (Saber et al. 2013). The
authors also observed elevated SAA3 protein levels in broncheoalveolar lavage fluid and
plasma after exposure to MWNT-7, whereas no changes in hepatic
Saa3
levels were
reported. Global gene expression analysis of pulmonary changes after exposure to a
small (L: 847 ±102 nm, D: 11 (6–17) nm) and a large MWCNT (L: 4048 ± 366 nm, D: 67
(24–138) nm) revealed that all
Saa
isoforms were among the most differentially regulated
genes 1, 3 and 28 days post-exposure (Poulsen et al. 2015b). Interestingly, both MWCNT
types also induced dose- and time-dependent increases in plasma protein SAA3 levels.
Increased SAA3 plasma protein levels have been reported in male mice exposed to a
pristine MWCNT (L: 15 ±5.0 µm and 13.5 ±1.50 nm) up to 1 year after intratracheal
exposure (Kim et al. 2015). This highlights the impact of pulmonary exposure to CNTs
on long-term vascular changes.
Changes in plasma SAA1/2 and SAA3 protein levels were investigated 1, 28 and 90 days
after pulmonary exposure to 14 commercial, well-characterized MWCNTs in female
C57BL/6J mice (Poulsen et al. 2017). In general, the majority of the MWCNTs induced
increases in both SAA1/2 and SAA3 plasma protein levels. No correlation was observed
between SAA1/2 and SAA3 plasma protein levels, which indicated that the changes are
controlled by different mechanisms. However, a very close correlation between
neutrophil influx and
Saa3
mRNA levels in lung tissue and SAA3 protein levels in blood
in mice have previously been reported (Poulsen et al. 2016;Saber et al. 2014), linking
inflammation and cardiovascular effects.
Conclusion
CNTs may promote atherosclerosis directly, by inducing accelerated plaque progression,
or indirectly, by inducing increased blood levels of acute phase response proteins. Dose-
response relationships have only sparsely been reported for CNT-induced increased
plaque progression, in part due to the low dynamic range of the assay, but also due to
study designs. In contrast, dose-response relationships have been established between
CNT exposure and increased levels of acute phase response proteins. However, the
studies reporting CNT-induced atherosclerotic effects have solely used pulmonary
deposition as exposure method, and thus, the studies cannot be used to establish OELs.
Due to the close interplay between inflammation, acute phase response and plaque
progression, inflammation as neutrophil influx can be used as a proxy for the acute
phase response. The current working group therefore considers cardiovascular effects as
a threshold effect that is regulated in parallel to inflammation.
30
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Reprotoxicity
A recent review summarized several studies of the potential for reproductive toxicity of
CNTs. Very few studies used the airway route of exposure (Ema et al. 2016). The review
concluded that there is potential for placental transfer of single- and multi-walled CNTs,
as reported in mouse studies following intravenous injection. Both SWCNTs and
MWCNTs were toxic to mouse fetuses when administered to the pregnant dam by
injection (intraperitoneal as well as intravenous). Oral gavage of MWCNTs induced no
developmental toxicity in mice and rats. In the following, only studies using airway
exposure to carbon nanotubes are reviewed. Sexually mature female mice received one
intratracheal instillation of 67 µg/mouse (~3.3 mg/kg) of MWCNTs (NM-400), one day
prior to cohabitation with a mature male. Pathological changes in lungs and liver were
observed in dams 6 weeks after instillation of the MWCNTs. The delivery of the first
litter was delayed by an average of 5 days in dams instilled with MWCNTs. No effects
were observed in gestation and litter parameters, offspring behavior in the open field or
startle test, or daily sperm production in the male offspring (DSP) (Hougaard et al. 2013).
To further characterize the delay in delivery of the first litter, estrous cycle regularity was
investigated by comparing vaginal smears before and after a single intratracheal
instillation of 67 µg of NM-400 MWCNT or vehicle. Reproductive function was analyzed
by measuring time to delivery of litters after instillation of 2, 18 or 67 µg of NM-400.
Exposure to MWCNT significantly prolonged the estrous cycle during which MWCNT
exposure took place compared to the pre-exposure estrous cycles. In contrast was the
estrous cycle immediately after the exposed cycle significantly shortened in MWCNT
exposed females. No consistent effects were seen on time to delivery of litter or other
gestational parameters. Litter parameters, such as litter size, sex ratio, implantations, and
implantation loss were not affected by exposure (Johansson et al. 2017).
MWCNTs suspended in 2% carboxymethyl cellulose were intratracheally instilled in
pregnant mice at 3, 4, or 5 mg/kg (Fujitani et al. 2012). At 4 and 5 mg/kg, exposed females
presented with reduced weight gain and significant increases in leucocyte counts in
peripheral blood. Fetal growth was significantly inhibited at 5 mg/kg. Data analysis
showed statistically significant increases in the number of litters with malformed fetuses,
at 4 and 5 mg/kg, owing mainly to increased incidences of fetuses with fusion of
vertebral bodies and arches. It should be noted that the vehicle used is unusual, and that
compared to control animals, the exposed females presented with 25 and 45% increases
in lung weight at 4 and 5 mg/kg, respectively. The finding warrants further
investigation, preferably exposing the pregnant animals by inhalation.
Conclusion
The findings in these studies indicate that CNTs may interfere with reproduction. The
applied route of instillation is intratracheal instillation in all three studies, and they can
therefore not be used as basis for derivation of occupational limit values.
31
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
M
ECHANISMS OF TOXICITY
Pulmonary inflammation
Following pulmonary exposure, CNTs interact with the cellular membranes of resident
cells and proteins in the lung environment. When sensing CNTs through scavenger
receptors and/or toll-like receptors, macrophages and epithelial cells start secreting pro-
inflammatory cytokines. Alternatively, macrophages can initiate the inflammatory
response after sensing damage recognition-associated molecular patterns released from
damaged epithelial cells interacting with CNTs.
The surface area of the deposited CNTs is of great important for their level of interaction
with the resident pulmonary cell and thus their inflammogenic potential. In a study
investigating neutrophil influx after intratracheal instillation to 10 different MWCNTs at
three different time points (1, 28 and 92 days post-exposure), 83% of the observed
variation in neutrophil influx was predicted by BET surface area of the delivered dose
(r
2
=0.69)(Poulsen et al. 2016). In multiple linear regression analysis with independent
physico-chemical properties, dose and BET surface area were the most consistent and
significant predictors of inflammation in terms of neutrophil influx)(Poulsen et al. 2016).
In agreement with this, particle-induced pulmonary inflammation was similarly
predicted by deposited surface area of low-toxicity-low solubility particles (Duffin et al.
2007;Schmid and Cassee 2017).
Identification of physico-chemical properties important for MWCNT-induced
inflammation, also revealed that MWCNT length and oxygen content (interpreted as
surface hydroxylation and carboxylation) affected the inflammatory potential of
MWCNTs (Poulsen et al. 2016). Length has previously been proposed as a determinant
of CNT-induced inflammation, albeit the strongest cases have been made after
intraperitoneal or pleural deposition (Murphy et al. 2011;Poland et al. 2008;Yamashita et
al. 2010). CNTs of a certain length (above 10 µm) can comply with the fiber paradigm
(Donaldson et al. 2011) resulting in frustrated phagocytosis due to the inability of
macrophages to completely engulf the long CNTs. This leads to lysosomal instability,
activation of the NALP inflammasome, and chronic stimulation of the cell, which results
in the macrophage releasing a range of pro-inflammatory molecules (Donaldson et al.
2011). Length could also be important for inflammogenicity of the vast types of CNTs
with lengths lesser than 10 µm. Kobler et al. reported that a thick, fiber-like MWCNT
appeared to escape vesicle enclosure more often compared to a smaller, entangled
MWCNT, which could lead to increased intracellular damage (Kobler et al. 2015).
However, the same two types of MWCNT induced comparable levels of neutrophil
influx up to 28 days after exposure by intratracheal instillation (Poulsen et al. 2015b). In
conclusion, the importance of length for CNT-induced inflammation is therefore largely
uncertain for CNTs with lengths below 10 µm.
A higher surface density of oxidized groups on the CNTs could increase their
hydrophilicity and dispersion in the lung. Supporting this, increased MWCNT oxygen
content (surface hydroxylation and carboxylation) was identified as protective of
inflammation 28 days after exposure to 10 different MWCNTs (Poulsen et al. 2016).
32
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Similar lowered or eliminated toxicity has previously been reported in the literature (Jain
et al. 2011;Sager et al. 2014). Hydrophilic CNTs may be cleared faster from the lung than
hydrophobic CNTs, as they more easily disperse in the lung environment. In addition,
the acidic treatment used to introduce the oxidized groups often introduces structural
defects in the CNTs. This renders the CNT more susceptible for enzymatic and oxidative
breakage, resulting in greater bio-degradability and thus more effective pulmonary
clearance (Liu et al. 2010).
In conclusion, CNTs are in general inflammogenic upon pulmonary exposure and their
surface area has been identified as important for the potency of the inflammatory
response. Inflammation is considered a threshold effect.
Genotoxicity and cancer
CNT-induced genotoxicity may occur by several mechanisms. These are briefly
described.
Surface-dependent ROS generation
Carbon-based nanomaterials such as carbon black are efficient generators of radicals
in
vitro
and in acellular assays (Jacobsen et al. 2008). SWCNT and MWCNTs also generate
ROS in acellular assays (Jackson et al. 2015;Jacobsen et al. 2008). ROS may result in
oxidation of the DNA, which can result in several types of DNA damage, including base
modifications, abasic sites, single-strand breaks, protein-DNA adducts, and DNA
crosslinks (Waris and Ahsan 2006). SWCNT-induced ROS is linked to generation of fpg-
sensitive sites
in vitro,
but did not affect the mutation frequency in the FE1 cell line
(Jacobsen et al. 2008).
Fiber-related DNA damage
Poulsen et al. (Poulsen et al. 2016) searched for physico-chemical predictors of DNA
strand break levels. Increasing MWCNT diameter was the most consistent and
significant predictor of DNA strand break levels in the comet assay. This supports a
mechanism related to the fibrous structure of thick MWCNTs. Similar to this, (Catalan et
al. 2016) compared genotoxicity of one entangled MWCNT and one straight MWCNT
(MWNT-7), and found that the straight MWCNT in contrast to the entangled MWCNT
induced DNA stand breaks in bronchoalveolar lavage cells and in lung tissue. The
straight MWCNT also induced micronuclei in alveolar type II cells, whereas this was not
assessed for the entangled MWCNT. The authors concluded that both primary and
secondary mechanisms may be involved in the observed genotoxicity of the straight
MWCNT.
The fiber paradigm as mechanism of CNT-induced carcinogenicity
Long CNTs have been proposed to induce gentoxicity according to the fiber paradigm
(Donaldson et al. 2011). Thus, macrophage interaction with long and straight MWCNTs
results in frustrated phagocytosis due to the inability of macrophages to completely
engulf the long CNTs. The slow clearance of insoluble MWCNT and frustrated
phagocytosis are the essential elements of the fiber paradigm (Donaldson et al. 2011).
33
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
In conclusion, the mechanism of CNT-induced cancer has not been firmly established
yet. With respect to DNA damage there is no evidence indicating for a threshold mode of
action for CNTs. The current working group therefore assumes a non-threshold mode of
action for CNT-induced carcinogenicity.
Cardiovascular effects
CNT exposure can lead to cardiovascular effects either: 1. Directly, by translocation of
CNTs from the lung to the vascular system. 2. Indirectly, as a consequence of pulmonary
inflammation. 3. Alterations in autonomic nervous system activity to elicit peripheral
effects.
Atherosclerosis is a central cardiovascular effect, which is manifested as increased
plaque deposition or build-up in the arteries. It is initiated by a biological, chemical or
physical insult to the artery walls. Translocated CNTs could induce this insult by
interacting directly with the endothelium. This leads to the expression of cell adhesion
molecules (selectins, VCAM-1 and ICAM-1) on the endothelial lining of the arteries,
which facilitates the activation, recruitment and migration of monocytes through the
endothelial monolayer (Hansson and Libby 2006;Cybulsky et al. 2001). Inside the intima
layer, the monocytes differentiate into macrophages and internalize fatty deposits
(mainly oxidized low density lipoprotein), transforming them into foam cells, which is a
major component of the atherosclerotic fatty streaks. The fatty streaks reduce the
elasticity of the artery walls and the foam cells promote a pro-inflammatory environment
by secretion of cytokines and ROS. In addition, foam cells also induce the recruitment of
smooth muscle cells to the intima. Added together, these changes lead to the formation
of plaques on the artery walls. A fibrous cap of collagen and vascular smooth muscle
cells protects the necrotic core and stabilizes the plaque (Libby 2002;Virmani et al. 2005).
Although initially asymptomatic, narrowing of the blood vessels can lead to other
cardiovascular diseases, such as coronary artery disease or stroke. In addition, blood
clots can be formed if the plaque ruptures. These may travel with the bloodstream and
obstruct the blood flow of smaller vessels.
Pulmonary exposure to CNTs may also promote accelerated atherosclerosis indirectly
through an induced pulmonary acute phase response. Introduction of CNTs to the lung
promotes neutrophil influx and release of proinflammatory cytokines, which leads to
increased production of SAA proteins. The SAAs are hydrophobic proteins that upon
secretion in their target organs are able to translocate to the blood. A statistically
significant correlation between Saa3 mRNA levels in the lung and SAA3 protein levels in
the blood have previously been reported (Poulsen et al. 2015a), indicating that SAA3
produced in the target organ translocate to systemic circulation. SAA circulating in the
blood becomes incorporated with HDL, thereby replacing Apolipoprotein A1 (Apo-A1)
as the major HDL-associated protein and forming HDL-SAA. The formation of HDL-
SAA has a double effect on plaque progression: 1.
HDL
is a major component of reverse
cholesterol transport, a multi-stepped process resulting in the movement of cholesterol
through the blood from peripheral tissues (including the artery walls) to the liver. The
formation of SAA-HDL impairs the HDL-mediated reverse cholesterol transport,
resulting in reduced cholesterol transport and an increased systemic total cholesterol
34
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
pool (Lindhorst et al. 1997;Steinmetz et al. 1989). 2. SAA and SAA-HDL have been
shown to directly stimulate the transformation of macrophages into foam cells and to
stimulate uptake of oxidized LDL in the macrophages (Lee et al. 2013). In addition, SAA-
HDL has a lower capacity to promote cellular cholesterol efflux from macrophages than
native HDL (Artl et al. 2000).
Identification of physico-chemical properties important for changes in plasma SAA
concentration indicated that increasing length predicts decreasing SAA1/2 plasma levels,
whereas metal content is important for SAA3 plasma levels (Poulsen et al. 2017). The
authors reported a significant correlation between plasma SAA3 levels and neutrophil
influx in the lung. Interestingly, pulmonary neutrophil influx has previously been shown
to correlate with deposited surface area of instilled MWCNT (Poulsen et al. 2016), which
links deposited CNT surface area with increased risk of developing cardiovascular
disease.
In conclusion, pulmonary exposure to CNTs can lead to accelerated plaque progression
directly, through translocation, or indirectly, through an induced acute phase response.
No single physico-chemical property has been identified as the driver of cardiovascular
effects, but CNT surface area a likely important due to the close connection to
pulmonary inflammation. As for inflammation, the current working group consider
cardiovascular effects as a threshold effect. This is based on identified dose-response
relationships between CNT exposure dose and induced acute phase response (Poulsen et
al. 2015a;Saber et al. 2013), and the close interplay between inflammation, acute phase
response and plaque progression. A very close correlation between neutrophil influx and
Saa3
mRNA levels in lung tissue and SAA3 protein levels in blood in mice were also
observed (Poulsen et al. 2016;Saber et al. 2014). Based on this, the current working group
suggests that inflammation in terms of neutrophil influx is used as biomarker of
cardiovascular risk.
Dose-response relationships
In general, dose response relationship is observed for health effects following pulmonary
exposure to CNTs.
Inflammation
Strong dose response relationships have consistently been observed for various markers
of pulmonary inflammation (Pauluhn 2010b;Ma-Hock et al. 2009;Pothmann et al.
2015;Kasai et al. 2015) and acute phase response (Poulsen et al. 2017;Saber et al.
2014;Saber et al. 2013;Poulsen et al. 2015b;Poulsen et al. 2015a). The working group
therefore considers inflammation as a threshold effect.
Cancer
Dose response relationship has been observed for carcinogenic effects of MWCNT in
several independent studies (Kasai et al. 2016;Rittinghausen et al. 2014;Takagi et al.
2012). The dose-response relationship was observed on mass-base for the individual
CNTs. However, in the study by (Rittinghausen et al. 2014) several different MWCNTs
35
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
were compared, and differences in the carcinogenic potential between the MWCNTs
could not be explained by mass or by the number of CNT fibers.
Genotoxicity by comet assay
No consistent dose-response relationship has been observed for CNT-induced DNA
strand breaks in the comet assay (Poulsen et al. 2016;Catalan et al. 2016;Poulsen et al.
2015b). However, the lack of dose-response relationship may reflect that the level of
CNT-induced DNA strand breaks is low, leading to a low dynamic range of the comet
assay for this application. The mechanisms of CNT-induced genotoxicity and
carcinogenicity are not fully clarified. It is therefore not possible to conclude on a
threshold mechanism and a non-threshold approach will be used as the most
conservative approach.
Plaque progression
Dose-response relationships are rarely reported for CNT-induced increased plaque
progression. This is in part due to the low dynamic range of the assay, but also due to the
study designs, which often only include one dose. The exception is the study by Suzuki
and colleagues, which reported that their high doses of SWCNT and DWCNT induced
increased plaque progression after pharyngeal aspiration, whereas the low doses did not
(Suzuki et al. 2016). Alternatively, the acute phase response proteins could be used as
relevant biomarkers for plaque progression. Dose-response relationships are established
between CNT exposure and increased levels of these proteins. The current working
group considers cardiovascular effects as a threshold effect that should be regulated in
parallel to inflammation.
36
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
P
REVIOUS RISK ASSESSMENTS OF CARBON
NANOTUBES
During the last couple of years, researchers, producers and organizations have proposed
recommended exposure limits, indicative or derived no-effect-level (INEL/DNEL) and
occupational exposure levels for CNTs. These have been set based on pulmonary
inflammation or lung cancer identified as the most critical effects of CNT exposure. The
previous recommendations of exposure limits are presented below and an overview can
be found in Table 4.
•
•
•
•
•
•
Aschberger et al. 2010, Critical Reviews in Toxicology (Aschberger et al. 2010).
Pauluhn 2010, Regulatory Toxicology and Pharmacology (Pauluhn 2010a).
ENRHES, Engineered Nanoparticles: Review of Health and Environmental
Safety, Final report, 2009 (ENRHES 2009).
NIOSH, 65, Occupational Exposure to Carbon Nanotubes and Nanofibers, 2013
(NIOSH 2013).
IARC, Monograph no. 111 (Grosse et al. 2014).
Ministry of Environment and Food of Denmark, Carbon nanotubes,
Environmental project No. 1805, 2015 (Jensen et al. 2015).
Aschberger et al. 2010
As part of a comprehensive review on carbon nanotube toxicity and exposure,
Aschberger and colleagues included an assessment of possible human exposure limits
(Aschberger et al. 2010). INELs were derived using the methodology suggested in the
REACH guidelines (chapter R.8 in ECHA, 2008), and were based on two sub-chronic
inhalation studies by (Pauluhn 2010b) and (Ma-Hock et al. 2009). In these studies a
NOAEC (Pauluhn 2010b) and a LOAEC (Ma-Hock et al. 2009) of 0.1 mg/m
3
were
identified for pulmonary inflammation after end of exposure in rats exposed 6 hour/day,
5 days/week for 13 weeks to two different MWCNTs (Baytubes and Nanocyl,
respectively). After applying default assessment factors, the authors estimated INELs of
2 µg/m
3
for Baytubes and 1 µg/m
3
for Nanocyl.
Pauluhn 2010
A study by Pauluhn from 2010 suggested an occupational exposure limit for Baytubes
MWCNTs (a thin, flexible MWCNT type) at 0.05 mg/m
3
(time-weighted average)
(Pauluhn 2010a). The author based his assessment on two previously published papers
by the same author, investigating toxicity and agglomeration of inhaled Baytubes
MWCNTs in rats (Pauluhn 2011;Pauluhn and Rosenbruch 2015). The author stated that,
in the case of the Baytubes MWCNTs, overload could be the trigger of the cascade of
events leading to lowered clearance and consequently increased MWCNT doses high
enough to trigger sustained pulmonary inflammation. The occupational exposure limit
in this study was calculated based on this mechanism. The author applied multiple
interspecies adjustments, but did not follow the REACH guidelines.
37
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
ENRHES
Some of the first to establish derived-no-effect levels (DNEL) for CNTs was within the
frames of the EU project Engineered Nanoparticles: Review of Health and
Environmental Safety (ENRHES 2009). At the time of the report, no carcinogenicity
studies were identified for CNT exposure. The derivation of a DNEL was therefore made
under the assumption of a threshold driven mechanism of CNT toxicity. The calculated
DNELs of 33.5 µg/m
3
for pulmonary inflammation and 0.67 µg/m
3
for systemic immune
effects were derived based on a sub-acute study by (Mitchell et al. 2007). The calculations
of DNELs followed the methodology suggested in the REACH guidelines.
NIOSH
In 2010, the National Institute for Occupational Safety and Health (NIOSH) proposed a
recommended exposure limit (REL) of 7 µg/m
3
of CNTs in air as an eight-hour, time-
weighted average, respirable mass concentration. However, this value was re-adjusted to
be 1 µg carbon/m3 in April, 2013 (NIOSH 2013).This states: "The NIOSH REL is expected
to reduce the risk for pulmonary inflammation and fibrosis. However, because of some
residual risk at the REL and uncertainty concerning chronic health effects, including
whether some types of CNTs may be carcinogenic, continued efforts should be made to
reduce exposures as much as possible". The analysis conducted by NIOSH was focused
on the types of CNT included in published research studies, and it primarily based is
deriviation on the sub-chronic, rat inhalation studies (Ma-Hock et al. 2009;Pauluhn
2010b).
IARC
In 2014, the International Agency for Research on Carcinogenicity (IARC) classified the
MWCNT MWNT-7 as possibly carcinogenic to humans (group 2B). In Denmark,
substances classified as group 1, 2A and 2B by IARC are considered carcinogenic.
MWCNTs other than MWNT-7 were not classifiable as to their carcinogenicity to
humans (Group 3). SWCNTs were not classifiable as to their carcinogenicity to humans
(Group 3). These classifications were based on: 1. Inadequate evidence in humans for the
carcinogenicity of CNTs. 2. Sufficient evidence in experimental animals for the
carcinogenicity of MWNT-7 MWCNTs. 3. Limited evidence in experimental animals for
the carcinogenicity of two types of MWCNTs with dimensions similar to MWNT-7. 4.
Inadequate evidence in experimental animals for the carcinogenicity of MWCNT other
than MWNT-7. 5. Inadequate evidence in experimental animals for the carcinogenicity of
SWCNTs.
38
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Table 4. List of studies proposing occupational exposure limits based on LOAEC or NOAEC data in animal studies
Study
Aschberger et al. 2010
Pauluhn 2010a
NIOSH 2013
ENRHES 2009
Pulmonary
Pulmonary
Systemic
Pulmonary
Critical effect
Pulmonary inflammation
inflammation and
inflammation
immune effects
inflammation
fibrotic events
Duration
Acute
Sub-chronic
Sub-chronic
Sub-chronic
Sub-acute
Sub-acute
Ellinger-
Ziegelbauer
Pauluhn 2010b/
Pauluhn 2010b/
Mitchell et al.
Mitchell et al.
Pauluhn 2010b
Key studies
and Pauluhn
Ma-Hock et al. 2009
Ma-Hock et al. 2009
2007
2007
2009
MWCNT,
MWCNT,
MWCNT,
MWCNT,
MWCNT,
CNT type
Baytubes and
MWCNT, Baytubes
Baytubes and
Shenzhen
Shenzhen
Baytubes
Nanocyl NC 7000
Nanocyl NC 7000
Nanotech
Nanotech
Risk determinant
LOAEC
NOAEC/LOAEC
NOAEC
NOAEC/LOAEC
NOAEC
LOAEC
3
3
3
3
3
Risk level in rodents
11 mg/m
0.1 mg/m
0.1 mg/m
0.1 mg/m
0.5 mg/m
0.3 mg/m
3
NOAEC
Corrected
5.5 mg/m
3 a
0.05 mg/m
3 a
0.05 mg/m
3
-
2.5 mg/m
3
0.15 mg/m
3
Corrected starting point
Default
3
b
-/2
b
-
-
-
3
f
assessment factors
Interspecies
2.5
c
2.5
c
-
-
2.5
c
2.5
c
Intraspecies
5
c
5
c
-
-
5
c
5
c
Uncertainty
Sub-chronic or
factors:
sub-acute to
-
2
c
-
-
6
e
6
e
chronic
Dose-response
issues:
LOAEC/NOAEC
extrapolation/
severity of effect
Overall uncertainty factor
Suggested OEL
-
37.5
d
150 µg/m
3
-
25
d
2 µg/m
3
/ 1 µg/m
3
-
-
-
-
-
50 µg/m
3
-
1 µg/m
3
75
33.5 µg/m
3
75
0.67 µg/m
3
39
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Table 4 description. a) Correction factors: 8 hour working hours and difference in
respiratory volume between animals in rest and workers at light activity. Corrected
L(N)OAEC
worker
for 8 working hours: (N(L)OAEC × 6 h/8 h). Correction for the
difference in respiratory volume between animals at rest and workers at light activity
(N(L)OAEC × 6.7 m
3
/10 m
3
). b) extrapolation from LOAEC to NOAEC. c) Interspecies
default factor: 2.5. Intraspecies default factor: 5 for workers/10 for public. Sub-chronic
to chronic default factor: 2. d) Altogether, overall assessment factors of 37.5 (3 × 2.5 × 5)
for acute and of 25 (2.5 × 5 × 2) and 50 (2 × 2.5 × 5 × 2), respectively, for chronic exposure
of workers. e) Sub-acute to chronic default factor: 6. f) Extrapolation from a LOAEC to
a NAEC (extrapolation factor of 3).
40
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
S
CIENTIFIC BASIS FOR AN OCCUPATIONAL
EXPOSURE LIMIT
Different methods exist for calculating occupational exposure limits. The choice of
method depends on the mode of action of the substance, and can fundamentally be split
up in two approaches: Threshold effects or non-threshold effects. The threshold effect
approach relies on the assumption that the organism can withstand a certain dose before
adverse effects occur, whereas the non-threshold effects approach relies on the
assumption that any exposure to the substance can result in adverse effects. In this
report, the current working group will calculate proposed occupational exposure limits
based both on threshold effects or non-threshold effects.
Calculations of exposure limits based on cancer as
non-threshold effect
Carcinogenicity via genotoxic mechanisms is generally considered as a non-threshold
effect. This applies for CNT-induced carcinogenicity, as no evidence for threshold effects
are available for the mechanism of action for carbon nanotube-induced carcinogenicity.
Risk levels are calculated based on the 2 year inhalation study by (Kasai et al. 2016). In
this study, MWNT-7 MWCNTs were used. MWNT-7 are relatively thick and long
MWCNTs (D: 92.9-98.2 nm, L: 5.8-5.9 µm) and have very low levels of chemical
contaminations compared to other MWCNTs. It is therefore unlikely that the observed
effects are attributed to metal contaminations. The MWCNTs were aerosolized to
generate single fibers.
Lung burden was measured in lung tissue. Tissue was digested and fibers were counted
on SEM images of digested tissue. Time and air concentration-dependent accumulation
of MWCNTs were observed during the entire 2-year study. Lung deposition was 1.5-
2.7% (Kasai et al. 2016). Male rats were more sensitive compared to female rats, and thus
the proposed occupational exposure limit was calculated based on cancer incidents in
males. The authors reported statistically significantly increased lung cancer incidence in
male rats at 0.2 mg/m
3
. Both malignant and non-malignant tumors were included (Table
5).
Table 5. Cancer incidents and lung burden in male rats after exposure to different
doses of MWCNT in (Kasai et al. 2016).
0 mg/m
3
0.02 mg/m
3
0.2 mg/m
3
2 mg/m
3
Total cancer
2/50
2/50
13/50
16/50
incidences
MWCNT lung
10
152.4
1797.8
burden (µg/lung)
41
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Method I
The current working group has chosen to use the approach used by (Kasai et al. 2016)
and (Erdely et al. 2013), who use the measured lung burden in rats exposed by inhalation
and the alveolar surface area of rats and humans to estimate the human equivalent lung
burden:
Observed cancer incidence at 0.2 mg/m
3
:
(13
0.2 mg
- 2
control
)/(50
control
- 2
control
) = 11/48 = 23%
Lung deposited dose in male rats at 0.2 mg/m
3
: 0.152 mg/lung.
The human equivalent dose is:
(Rat deposited dose) x (human alveolar surface area)/(rat alveolar surface area) =
0.152 mg x 102 m
2
/0.4 m
2
= 38.76 mg MWCNT per human lung.
The following standardized constants are assumed:
The standard value of human ventilation is 20 L/min during light work (1.2 m
3
/h).
An average work day is 8h per day.
An average work week contains 5 days.
In Denmark, an average employee work 45 weeks per year.
An average working life is 45 years.
The pulmonary deposition rate was reported to be 1.4-2.7% in the study Kasai and
colleagues (Kasai et al. 2016). However, in a 13 week inhalation study by Pauluhn, a
pulmonary deposition rate of 5.7 % was reported for aerosolized agglomerated
MWCNTs (Pauluhn 2010b). In order to take different kinds of MWCNTs as well as
different states of agglomeration into account, the current working group use the
pulmonary deposition rate of 5.7% as reported by (Pauluhn 2010b).
Using the values above, a lung burden of 38.8 mg in humans would require that workers
are exposed to:
Air concentration =
38.76 mg/(8h/day x 5 day/week x 45 weeks/year x 45 years x 1.2 m
3
/h x 0.057) =
0.00699 mg/m
3
.
Thus, at an air concentration of 7 µg/m
3
during a 45 year work life, an excess lung cancer
incidence of 23% is expected. Assuming a non-threshold linear dose-response
relationship, then 1% excess lung cancer is expected at:
(7 µg/m
3
)/23 = 0.304 µg/m
3
42
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Table 6. Calculated excess lung cancer incidence at different MWCNT mass and fiber
concentrations for 8 hour daily occupational exposure, Method I.
Excess lung cancer
MWCNT Air
Air concentration
incidence
concentration (µg/m
3
) MWNT-7 fibers/cm
3
*
1:1,000
0.03
0.3
1: 10,000
0.003
0.03
1: 100,000
0.0003
0.003
*) The calculations of fiber concentrations were based on the following: 1 µg MWNT-7
MWCNT corresponds to 9.03 x 10
6
MWNT-7 fibers (Kasai et al. 2016).
Only MWNT-7 has been shown to be carcinogenic by inhalation. One other type of
MWCNT did not induce cancer in a 2 year study (Muller et al. 2009). However, several
types of MWCNTs have been shown to be carcinogenic by other exposure routes
(Rittinghausen et al. 2014) or genotoxic (Poulsen et al. 2016;Catalan et al. 2016;Poulsen et
al. 2015b). Moreover, characterization of the physico-chemical properties of
commercially available CNTs has shown large deviations between empirical data and
data available from the supplier (Jackson et al. 2015). The present working group
therefore assume that all CNTs are carcinogenic.
Method II
Calculation based on approach suggested by ECHA (ECHA
2012;SCHER/SCCP/SCENIHR 2009), calculated based on the one year MWCNT
inhalation study in rats by (Kasai et al. 2016)(Table 5):
Excess cancer risk:
(13
0.2 mg
- 2
control
)/(50
control
- 2
control
) = 11/48 = 23%
Correction of dose metric into a human dose situation (8h/d):
0.2 mg/m
3
x (6h/day)/(8h/day) x (6.7 m
2
/10 m
2
) = 0.1 mg/m
3
or 100 µg/m
3
Calculation of unit risk for cancer:
Risk level = exposure level x unit risk
0.23 = 100 µg/m
3
x unit risk
Unit risk = 2.3 x 10
-3
per µg/m
3
At a dose of 1 µg/m
3
, 2.3 x 10
-3
excess cancers are expected.
Calculation of dose levels corresponding to risk level of 10
-5
(and other risk levels)
10
-5
risk level = exposure level x unit risk (2.3 x 10
-3
per µg/m
3
)
Exposure level (10
-5
) = 4.3 x 10
-3
µg/m
3
43
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Table 7. Calculated excess lung cancer incidence at different MWCNT mass
concentrations for 8 hour daily occupational exposure, Method II.
Excess lung cancer
MWCNT Air
incidence
concentration (µg/m
3
)
1:1,000
0.043
1: 10,000
0.0043
1: 100,000
0.00043
Calculations of exposure limits based on
inflammation as threshold effect
Several studies have reported dose-dependent inflammatory effects as well as dose-
dependent acute phase response, a biomarker of risk of cardiovascular disease.
Inflammation, acute phase response and cardiovascular effects are therefore considered
threshold effects. However, at present only inflammogenicity has been investigated in
inhalation studies of sufficient quality for risk assessment. The current working group
therefore bases the risk assessment of threshold effects on the sub-chronic inhalation
studies investigating inflammogenicity. The choice of using the sub-chronic studies (Ma-
Hock et al. 2009;Pauluhn 2010b;Pothmann et al. 2015) , compared to the chronic one
(Kasai et al. 2016) is based on the CNT type being investigated (Table 2). In the sub-
chronic studies, the short, thin MWCNTs with large surface areas are used, whereas in
the chronic study it is the long, thick MWCNT type with small surface area. As described
in
Mechanism of toxicity,
CNT-induced inflammation has been shown to be predicted by
the specific surface area. In order to take the variation specific surface area into account
and as a precaution, the current working group has therefore decided to base the risk
assessment on the data from the sub-chronic studies.
NOAECs were noted for the highest air concentrations, which do not induce statistically
significantly increased levels of neutrophils (Table 2) (Ma-Hock et al. 2009;Pauluhn
2010a;Pauluhn 2010b;Pothmann et al. 2015). The lowest NOAEC for neutrophil influx
was reported by (Pothmann et al. 2015) to be 0.05 mg/m
3
. This is similar to the NOAECs
reported in other studies, albeit approximately half the concentration (Table 2). As a
precaution, the NOAEC of 0.05 mg/m
3
will be used for determining the proposed
occupational exposure limits for threshold effects.
Calculation
The DNEL for CNT-induced pulmonary inflammation is established based on the
methodology suggested in the REACH guidelines (ECHA 2012). Initially, a corrected
NOAEC is made that takes into account that rats were exposed for 6 h/day, whereas
humans are exposed for 8 h/day. In addition, exposed rats are at rest, whereas workers
are considered to do light activity. A total breathing volume of 10 m³ for an 8-hour shift
at light activity is therefore assumed, compared to 6.7 m
3
at rest.
Corrected NOAEC:
0.05 mg/m
3
x (6h/day)/(8h/day) x (6.7 m
2
/10 m
2
) = 0.0251 mg/m
3
44
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Next, assessment factors are applied. As no valid argumentation for using alternative
assessment factors could be found, the current working group uses the assessment
factors as proposed by ECHA (ECHA 2012):
Extrapolation from sub-chronic to chronic exposure: 2
Neutrophil influx is considered an acute response and this would normally not warrant
a duration factor from a sub-chronic study. However the accumulation of CNTs over
time would continue beyond sub-chronic exposure and thus the default assessment
factor of 2 to extrapolate from sub-chronic to chronic exposure were chosen.
Extrapolation from rats to humans: 2.5
Variation between workers: 5
Total assessment factor: 2 x 2.5 x 5 = 25
Suggested exposure limit based on inflammation:
0.0251 mg/m
3
/25 (total assessment factor) = 0.001 mg/m
3
= 1 µg/m
3
.
45
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
C
ONCLUSION
The present working group evaluated the relevant literature on CNT toxicity from both
epidemiological and animal inhalation studies. However, as almost no human data on
toxicity and epidemiological studies is available, inhalation studies in mice and rats were
used to assess potential human hazard.
Carbon nanotubes are a very diverse class of nanomaterials with large variation in
physico-chemical properties including diameter, length, specific surface area, level and
type of contaminations, surface modifications. Therefore, as the relationship between
physico-chemical properties of CNTs and their inhalation toxicity is not fully clarified,
the present working group considers all types of CNTs a respiratory hazard and
proposes to regulate all CNTs as one group.
The present working group regards inflammation and carcinogenicity as the main
adverse effects of CNT exposure by inhalation and the subsequent risk assessments are
conducted based on studies reporting these effects. CNT-induced cardiovascular effects
were also identified in animal studies. But as none of these studies were sub-chronic or
chronic inhalation studies, they were not suitable for risk assessment. However, the
current working group regards the acute-phase response as a biomarker of
cardiovascular effects. Due to the close association between pulmonary inflammation
and the acute phase response, the current working group regards inflammation as a
proxy for cardiovascular effects.
The present working group found that the mechanism of action of CNT-induced
carcinogenic effect has not been fully clarified. CNTs have reported to induce ROS
generation similar to carbon black. ROS may result in oxidation of the DNA, which can
result in several types of DNA damage, including base modifications, abasic sites, single-
strand breaks, protein-DNA adducts, and DNA crosslinks (Waris and Ahsan 2006).
CNTs may also induce genotoxicity through they fibrous shape, both in regards to
diameter thickness (Poulsen et al. 2016;Catalan et al. 2016) and length (Donaldson et al.
2011). In addition, secondary genotoxicity due to CNT-induced inflammation has been
recognized as an important and well-documented mechanism of action for the
development of lung cancer. Based on the above mentioned findings, the current
working group did not find sufficient evidence for a threshold mechanism for CNT-
induced carcinogenicity and decided to consider it as non-threshold effect.
In contrast to cancer, the mechanism of action for CNT-induced pulmonary
inflammation is more established and the present working group found strong dose
response relationships for various markers of pulmonary inflammation, including
neutrophil influx (Kasai et al. 2015;Ma-Hock et al. 2009;Pauluhn 2010b;Pothmann et al.
2015). Neutrophil influx was predicted by deposited surface area. The working group
considers inflammation as a threshold effect.
Based on the main adverse effects of pulmonary CNT exposure, inflammation and
cancer, the working group decided to perform the risk assessment based on both a
threshold and a non-threshold mechanism of action. Four sub-chronic and one chronic
46
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
study inhalation study in rats were identified as suitable for determining a DNEL for
pulmonary inflammation. A conservation approach was selected and the DNEL was
calculated based on the study using the CNT with the largest specific surface area and
reporting the lowest NOAEC estimate (Pothmann et al. 2015)(Table 8). For the non-
threshold approach on cancerous effects, a 2 year inhalation study in rats were identified
as suitable (Kasai et al. 2016) and excess cancer risks at the levels of 1:1,000, 1:10,000 and
1 in 100,000 were calculated using two approaches (Table 8).
Table 8. Overview of DNEL based on a threshold based mechanism of action and
exposure levels resulting in excess cancer risk levels at 1:1000, 1:10 000 and 1: 100 000
based on a non-threshold based mechanism of action
.
Suggestion for an OEL for CNTs
Lung cancer
Inflammation
(Method I)
DNEL
1 µg/m
3
Excess cancer risk:
1:1,000
0.03 µg/m
3
1:10,000
0.003 µg/m
3
1:100,000
0.0003 µg/m
3
Mechanism of action
Threshold based
Non-threshold based
Lung cancer
(Method II)
0.043 µg/m
3
0.0043 µg/m
3
0.00043 µg/m
3
The present working group regards cancer as the most critical effect. Two different
approaches were used for calculating excess lung cancer risk based on the same chronic
inhalation study (Kasai et al. 2016). In the first approach, lung burden was used to
estimate the exposure levels. In the second approach, air concentrations were used
directly. Independently of the applied method for risk assessment, the acceptable
exposure levels were all very low. These levels are all more than 5 magnitudes lower
than the present Danish occupational exposure limit for bulk carbon black of 3.5 mg/m
3
.
The present working group recommends the approach using the excess lung cancer risk
estimates based on lung burden, since this approach takes the retained pulmonary dose
into account. Thus, the expected excess lung cancer risk based on lung burden approach
is 1:1,000 at 0.03 µg/m
3
, 1:10,000 at 0.003 µg/m
3
and 1:100,000 at 0.0003 µg/m
3
.
47
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
R
EFERENCES
Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of
acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol 2000;20:763-772.
Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SA, Tran CL, Christensen FM.
Review of carbon nanotubes toxicity and exposure--Appraisal of human health risk assessment
based on open literature. Crit Rev Toxicol 2010;40:759-790.
Baisch BL, Corson NM, Wade-Mercer P, Gelein R, Kennell AJ, Oberdörster G, Elder A. Equivalent
titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation:
The effect of dose rate on acute respiratory tract inflammation. Part Fibre Toxicol 2014;11:5.
Balarak D, Mahdavi Y, Maleki A, Daraei H, Sadeghi S. Studies on the removal of amoxicillin by
single walled carbon nanotubes. British Journal of Pharmaceutical Research 2016;10:1-9.
Birch ME, Ku BK, Evans DE, Ruda-Eberenz TA. Exposure and emissions monitoring during
carbon nanofiber production-Part I: Elemental carbon and iron-soot aerosols. Ann Occup Hyg
2011;55:1016-1036.
Bourdon JA, Halappanavar S, Saber AT, Jacobsen NR, Williams A, Wallin H, Vogel U, Yauk CL.
Hepatic and pulmonary toxicogenomic profiles in mice intratracheally instilled with carbon
black nanoparticles reveal pulmonary inflammation, acute phase response, and alterations in
lipid homeostasis. Toxicol Sci 2012a;127:474-484.
Bourdon JA, Saber AT, Jacobsen NR, Jensen KA, Madsen AM, Lamson JS, Wallin H, Míller P, Loft
S, Yauk CL, Vogel U. Carbon black nanoparticle instillation induces sustained inflammation and
genotoxicity in mouse lung and liver. Part Fibre Toxicol 2012b;9:5.
Bourdon JA, Williams A, Kuo B, Moffat I, White PA, Halappanavar S, Vogel U, Wallin H, Yauk
CL. Gene expression profiling to identify potentially relevant disease outcomes and support
human health risk assessment for carbon black nanoparticle exposure. Toxicology 2013;303:83-93.
Calero C, Arellano E, Lopez-Villalobos JL, Sanchez-Lopez V, Moreno-Mata N, Lopez-Campos JL.
Differential expression of C-reactive protein and serum amyloid A in different cell types in the
lung tissue of chronic obstructive pulmonary disease patients. BMC Pulm Med 2014;14:95.
Cancer Risks UK. Worldwide cancer mortality statistics. Cancer Risks UK, 2018.
https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-
cancer/mortality
Cao Y, Jacobsen NR, Danielsen PH, Lenz AG, Stoeger T, Loft S, Wallin H, Roursgaard M,
Mikkelsen L, Míller P. Vascular effects of multiwalled carbon nanotubes in dyslipidemic ApoE-/-
mice and cultured endothelial cells. Toxicol Sci 2014;138:104-116.
Catalan J, Siivola KM, Nymark P, Lindberg H, Suhonen S, Jarventaus H, Koivisto AJ, Moreno C,
Vanhala E, Wolff H, Kling KI, Jensen KA, Savolainen K, Norppa H. In vitro and in vivo genotoxic
effects of straight versus tangled multi-walled carbon nanotubes. Nanotoxicology 2016;10:794-806.
48
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Christophersen DV, Jacobsen NR, Andersen MH, Connell SP, Barfod KK, Thomsen MB, Miller
MR, Duffin R, Lykkesfeldt J, Vogel U, Wallin H, Loft S, Roursgaard M, Møller P. Cardiovascular
health effects of oral and pulmonary exposure to multi-walled carbon nanotubes in ApoE-
deficient mice. Toxicology 2016;371:29-40.
Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med 2009;59:517-
526.
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly
PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin
Invest 2001;107:1255-1262.
Czarny B, Georgin D, Berthon F, Plastow G, Pinault M, Patriarche G, Thuleau A, L'Hermite MM,
Taran F, Dive V. Carbon nanotube translocation to distant organs after pulmonary exposure:
Insights from in situ (14)C-radiolabeling and tissue radioimaging. ACS Nano 2014;8:5715-5724.
Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Fernback JE. Occupational exposure
assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup
Hyg 2012;56:542-556.
Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon
nanotube and nanofiber exposure assessments: An analysis of 14 site visits. Ann Occup Hyg
2015;59:705-723.
De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: Present and future
commercial applications. Science 2013;339:535-539.
Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard
of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine (Lond)
2011;6:143-156.
Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural
mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal
pleura, inflammation and mesothelioma. Part Fibre Toxicol 2010;7:5.
Dresselhaus MS, Dresselhaus G, Charlier JC, Hernandez E. Electronic, thermal and mechanical
properties of carbon nanotubes. Philos Trans A Math Phys Eng Sci 2004;362:2065-2098.
Duffin R, Tran L, Brown D, Stone V, Donaldson K. Proinflammogenic effects of low-toxicity and
metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface
reactivity. Inhal Toxicol 2007;19:849-856.
ECHA. Guidance on information requirements and chemical safety assessment. Appendix R8-15
Recommendations for nanomaterials applicable to Chapter R.8 Characterisation of dose
[concentration] - response for human health. ECHA-12-G-09-EN. European Chemicals Agency,
2012.
ECHA. Guidance on information requirements and chemical safety assessment Chapter R.8:
Characterisation of dose [concentration]-response for human health. ECHA-2010-G-19-EN.
European Chemicals Agency, 2012.
49
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Ellinger-Ziegelbauer H, Pauluhn J. Pulmonary toxicity of multi-walled carbon nanotubes
(Baytubes) relative to alpha-quartz following a single 6h inhalation exposure of rats and a 3
months post-exposure period. Toxicology 2009;266:16-29.
Ema M, Hougaard KS, Kishimoto A, Honda K. Reproductive and developmental toxicity of
carbon-based nanomaterials: A literature review. Nanotoxicology 2016;10:391-412.
ENRHES. Engineered Nanoparticles: Review of Health and Environmental Safety, 2009.
https://www.nanowerk.com/nanotechnology/reports/reportpdf/report133.pdf
Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML,
Deddens JA, Hulderman T, Bilgesu SA, Battelli L, Schwegler-Berry D, Leonard HD, McKinney W,
Frazer DG, Antonini JM, Porter DW, Castranova V, Schubauer-Berigan MK. Carbon nanotube
dosimetry: From workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol
2013;10:53.
Erdely A, Hulderman T, Salmen R, Liston A, Zeidler-Erdely PC, Schwegler-Berry D, Castranova
V, Koyama S, Kim YA, Endo M, Simeonova PP. Cross-talk between lung and systemic circulation
during carbon nanotube respiratory exposure. Potential biomarkers. Nano Lett 2009;9:36-43.
Fatkhutdinova LM, Khaliullin TO, Vasil'yeva OL, Zalyalov RR, Mustafin IG, Kisin ER, Birch ME,
Yanamala N, Shvedova AA. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl
Pharmacol 2016;299:125-131.
Fujitani T, Ohyama K, Hirose A, Nishimura T, Nakae D, Ogata A. Teratogenicity of multi-wall
carbon nanotube (MWCNT) in ICR mice. J Toxicol Sci 2012;37:81-89.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl
J Med 1999;340:448-454.
Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El GF, Bouvard V, brahim-Tallaa L, Guha N,
Scoccianti C, Mattock H, Straif K. Carcinogenicity of fluoro-edenite, silicon carbide fibres and
whiskers, and carbon nanotubes. Lancet Oncol 2014;15:1427-1428.
Halappanavar S, Jackson P, Williams A, Jensen KA, Hougaard KS, Vogel U, Yauk CL, Wallin H.
Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute
phase response genes, inflammatory cascades, and changes in microRNAs: A toxicogenomic
study. Environ Mol Mutagen 2011;52:425-439.
Hamilton RF, Jr., Wu Z, Mitra S, Shaw PK, Holian A. Effect of MWCNT size, carboxylation, and
purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxicol
2013;10:57.
Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, Lee SB, Ji JH, Cho MH, Yu IJ. Monitoring
multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol
2008;20:741-749.
Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev
Immunol 2006;6:508-519.
50
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Hedmer M, Isaxon C, Nilsson PT, Ludvigsson L, Messing ME, Genberg J, Skaug V, Bohgard M,
Tinnerberg H, Pagels JH. Exposure and emission measurements during production, purification,
and functionalization of arc-discharge-produced multi-walled carbon nanotubes. Annals of
Occupational Hygiene 2014;58:355-379.
Hougaard KS, Jackson P, Kyjovska ZO, Birkedal RK, De Temmerman PJ, Brunelli A, Verleysen E,
Madsen AM, Saber AT, Pojana G, Mast J, Marcomini A, Jensen KA, Wallin H, Szarek J, Mortensen
A, Vogel U. Effects of lung exposure to carbon nanotubes on female fertility and pregnancy. A
study in mice. Reprod Toxicol 2013;41:86-97.
Husain M, Saber AT, Guo C, Jacobsen NR, Jensen KA, Yauk CL, Williams A, Vogel U, Wallin H,
Halappanavar S. Pulmonary instillation of low doses of titanium dioxide nanoparticles in mice
leads to particle retention and gene expression changes in the absence of inflammation. Toxicol
Appl Pharmacol 2013;269:250-262.
Iijima S. Helical microtubules of graphitic carbon. Nature 1991;354:56-58.
Ismail AF, Goh PS, Tee JC, Sanip SM, Aziz M. A review of purification techniques for carbon
nanotubes. NANO: Brief Reports and Reviews 2018;3:127-143.
Jackson P, Halappanavar S, Hougaard KS, Williams A, Madsen AM, Lamson JS, Andersen O,
Yauk C, Wallin H, Vogel U. Maternal inhalation of surface-coated nanosized titanium dioxide
(UV-Titan) in C57BL/6 mice: Effects in prenatally exposed offspring on hepatic DNA damage and
gene expression. Nanotoxicology 2013;7:85-96.
Jackson P, Kling K, Jensen KA, Clausen PA, Madsen AM, Wallin H, Vogel U. Characterization of
genotoxic response to 15 multiwalled carbon nanotubes with variable physicochemical properties
including surface functionalizations in the FE1-Muta(TM) mouse lung epithelial cell line. Environ
Mol Mutagen 2015;56:183-203.
Jacobsen NR, Pojana G, White P, Moller P, Cohn CA, Korsholm KS, Vogel U, Marcomini A, Loft S,
Wallin H. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled
carbon nanotubes and C(60) fullerenes in the FE1-Mutatrade markMouse lung epithelial cells.
Environ Mol Mutagen 2008;49:476-487.
Jain S, Thakare VS, Das M, Godugu C, Jain AK, Mathur R, Chuttani K, Mishra AK. Toxicity of
multiwalled carbon nanotubes with end defects critically depends on their functionalization
density. Chem Res Toxicol 2011;24:2028-2039.
Jensen KA, Bøgelund J, Jackson P, Jacobsen NR, Birkedal R, Clausen PA, Saber AT, Wallin H,
Vogel U. Carbon nanotubes- Types, products, market, and provisional assessment of the
associated risks to man and the environment. Environmental project No. 1805. Copenhagen: The
Danish Environmental Protection Agency, 2015.
Johansson HKL, Hansen JS, Elfving B, Lund SP, Kyjovska ZO, Loft S, Barfod KK, Jackson P, Vogel
U, Hougaard KS. Airway exposure to multi-walled carbon nanotubes disrupts the female
reproductive cycle without affecting pregnancy outcomes in mice. Part Fibre Toxicol 2017;14:17.
Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey
Merz CN, Sopko G, Olson MB, Reis SE. Serum amyloid A as a predictor of coronary artery
disease and cardiovascular outcome in women: The National Heart, Lung, and Blood Institute-
Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:726-732.
51
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Kasai T, Umeda Y, Ohnishi M, Kondo H, Takeuchi T, Aiso S, Nishizawa T, Matsumoto M,
Fukushima S. Thirteen-week study of toxicity of fiber-like multi-walled carbon nanotubes with
whole-body inhalation exposure in rats. Nanotoxicology 2015;9:413-422.
Kasai T, Umeda Y, Ohnishi M, Mine T, Kondo H, Takeuchi T, Matsumoto M, Fukushima S. Lung
carcinogenicity of inhaled multiwalled carbon nanotube in rats. Part Fibre Toxicol 2016;13:53.
Kim JE, Lee S, Lee AY, Seo HW, Chae C, Cho MH. Intratracheal exposure to multi-walled carbon
nanotubes induces a nonalcoholic steatohepatitis-like phenotype in C57BL/6J mice.
Nanotoxicology 2015;9:613-623.
Kim JS, Sung JH, Choi BG, Ryu HY, Song KS, Shin JH, Lee JS, Hwang JH, Lee JH, Lee GH, Jeon K,
Ahn KH, Yu IJ. In vivo genotoxicity evaluation of lung cells from Fischer 344 rats following 28
days of inhalation exposure to MWCNTs, plus 28 days and 90 days post-exposure. Inhal Toxicol
2014;26:222-234.
Kinaret P, Ilves M, Fortino V, Rydman E, Karisola P, Lahde A, Koivisto J, Jokiniemi J, Wolff H,
Savolainen K, Greco D, Alenius H. Inhalation and oropharyngeal aspiration exposure to rod-like
carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs.
ACS Nano 2017;11:291-303.
Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A:
Mechanisms. Int J Cancer 2004;109:799-809.
Kobler C, Poulsen SS, Saber AT, Jacobsen NR, Wallin H, Yauk CL, Halappanavar S, Vogel U,
Qvortrup K, Mølhave K. Time-dependent subcellular distribution and effects of carbon nanotubes
in lungs of mice. PLoS One 2015;10:e0116481.
Lee HY, Kim SD, Baek SH, Choi JH, Cho KH, Zabel BA, Bae YS. Serum amyloid A stimulates
macrophage foam cell formation via lectin-like oxidized low-density lipoprotein receptor 1
upregulation. Biochem Biophys Res Commun 2013;433:18-23.
Lee JH, Lee SB, Bae GN, Jeon KS, Yoon JU, Ji JH, Sung JH, Lee BG, Lee JH, Yang JS, Kim HY, Kang
CS, Yu IJ. Exposure assessment of carbon nanotube manufacturing workplaces. Inhal Toxicol
2010;22:369-381.
Lee JS, Choi YC, Shin JH, Lee JH, Lee Y, Park SY, Baek JE, Park JD, Ahn K, Yu IJ. Health
surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology
2015;9:802-811.
Li Z, Hulderman T, Salmen R, Chapman R, Leonard SS, Young SH, Shvedova A, Luster MI,
Simeonova PP. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes.
Environ Health Perspect 2007;115:377-382.
Libby P. Inflammation in atherosclerosis. Nature 2002;420:868-874.
Lindhorst E, Young D, Bagshaw W, Hyland M, Kisilevsky R. Acute inflammation, acute phase
serum amyloid A and cholesterol metabolism in the mouse. Biochim Biophys Acta 1997;1339:143-
154.
Liu X, Hurt RH, Kane AB. Biodurability of single-walled carbon nanotubes depends on surface
functionalization. Carbon N Y 2010;48:1961-1969.
52
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: an
overview. Ann Periodontol 2001;6:1-8.
Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van
Ravenzwaay B, Landsiedel R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed
for 3 months. Toxicol Sci 2009;112:468-481.
Markets and Markets. Carbon Nanotubes (CNT) Market by Type (Single, Multi Walled), Method
(Chemical Vapor Deposition, Catalytic Chemical Vapor Deposition, High Pressure Carbon
Monoxide), Application (Electronics, Chemical, Batteries, Energy, Medical) - Global Forecast to
2022. Markets and Markets, 2017.
https://www.marketsandmarkets.com/Market-Reports/carbon-nanotubes-139.html
Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon
nanotube material: Aerosol release during the handling of unrefined single-walled carbon
nanotube material. J Toxicol Environ Health A 2004;67:87-107.
Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V,
Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon
nanotubes. Part Fibre Toxicol 2010;7:28.
Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter
DW. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol
2013;10:38.
Methner M, Beaucham C, Crawford C, Hodson L, Geraci C. Field application of the Nanoparticle
Emission Assessment Technique (NEAT): Task-based air monitoring during the processing of
engineered nanomaterials (ENM) at four facilities. J Occup Environ Hyg 2012;9:543-555.
Methner M, Hodson L, Dames A, Geraci C. Nanoparticle Emission Assessment Technique
(NEAT) for the identification and measurement of potential inhalation exposure to engineered
nanomaterials--Part B: Results from 12 field studies. J Occup Environ Hyg 2010;7:163-176.
Mezaki T, Matsubara T, Hori T, Higuchi K, Nakamura A, Nakagawa I, Imai S, Ozaki K, Tsuchida
K, Nasuno A, Tanaka T, Kubota K, Nakano M, Miida T, Aizawa Y. Plasma levels of soluble
thrombomodulin, C-reactive protein, and serum amyloid A protein in the atherosclerotic
coronary circulation. Jpn Heart J 2003;44:601-612.
Mikkelsen L, Sheykhzade M, Jensen KA, Saber AT, Jacobsen NR, Vogel U, Wallin H, Loft S,
Møller P. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone
mice exposed to nanosized TiO(2). Part Fibre Toxicol 2011;8:32.
Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and systemic
immune response to inhaled multiwalled carbon nanotubes. Toxicol Sci 2007;100:203-214.
Monse C, Hagemeyer O, Raulf M, Jettkant B, van K, V, Kendzia B, Gering V, Kappert G, Weiss T,
Ulrich N, Marek EM, Bunger J, Bruning T, Merget R. Concentration-dependent systemic response
after inhalation of nano-sized zinc oxide particles in human volunteers. Part Fibre Toxicol
2018;15:8.
53
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Morimoto Y, Hirohashi M, Kobayashi N, Ogami A, Horie M, Oyabu T, Myojo T, Hashiba M,
Mizuguchi Y, Kambara T, Lee BW, Kuroda E, Shimada M, Wang WN, Mizuno K, Yamamoto K,
Fujita K, Nakanishi J, Tanaka I. Pulmonary toxicity of well-dispersed single-wall carbon
nanotubes after inhalation. Nanotoxicology 2012a;6:766-775.
Morimoto Y, Hirohashi M, Ogami A, Oyabu T, Myojo T, Todoroki M, Yamamoto M, Hashiba M,
Mizuguchi Y, Lee BW, Kuroda E, Shimada M, Wang WN, Yamamoto K, Fujita K, Endoh S,
Uchida K, Kobayashi N, Mizuno K, Inada M, Tao H, Nakazato T, Nakanishi J, Tanaka I.
Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and
intratracheal instillation. Nanotoxicology 2012b;6:587-599.
Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to
multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci
2009;110:442-448.
Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, Prina-Mello
A, Volkov Y, Li S, Mather SJ, Bianco A, Prato M, MacNee W, Wallace WA, Kostarelos K,
Donaldson K. Length-dependent retention of carbon nanotubes in the pleural space of mice
initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol
2011;178:2587-2600.
Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K,
Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y,
Shinohara H, Toyokuni S. Diameter and rigidity of multiwalled carbon nanotubes are critical
factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A 2011;108:E1330-E1338.
Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of
all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 1994;14:133-140.
NIOSH. Occupational exposure to carbon nanotubes and nanofibers. Current Intelligence Bulletin
65. Cincinnati, OH: Department of Health and Human Services; Centers for Disease Control and
Prevention; National Institute for Occupational Safety and Health, 2013.
Ono-Ogasawara M, Takaya M, Yamada M. Exposure assessment of MWCNTs in their life cycle.
Journal of Physics: Conference Series 2015;617.
Pauluhn J. Multi-walled carbon nanotubes (Baytubes): Approach for derivation of occupational
exposure limit. Regul Toxicol Pharmacol 2010a;57:78-89.
Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic
effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol Sci
2010b;113:226-242.
Pauluhn J. Poorly soluble particulates: Searching for a unifying denominator of nanoparticles and
fine particles for DNEL estimation. Toxicology 2011;279:176-188.
Pauluhn J, Rosenbruch M. Lung burdens and kinetics of multi-walled carbon nanotubes
(Baytubes) are highly dependent on the disaggregation of aerosolized MWCNT. Nanotoxicology
2015;9:242-252.
Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest 2003;111:1805-1812.
54
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, MacNee
W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-
like pathogenicity in a pilot study. Nat Nanotechnol 2008;3:423-428.
Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram
K, Leonard S, Andrew M, Willard P, Tsuruoka S, Endo M, Tsukada T, Munekane F, Frazer DG,
Castranova V. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes.
Nanotoxicology 2013;7:1179-1194.
Pothmann D, Simar S, Schuler D, Dony E, Gaering S, Le Net JL, Okazaki Y, Chabagno JM,
Bessibes C, Beausoleil J, Nesslany F, Régnier JF. Lung inflammation and lack of genotoxicity in
the comet and micronucleus assays of industrial multiwalled carbon nanotubes
Graphistrength
©
C100 after a 90-day nose-only inhalation exposure of rats. Part Fibre Toxicol 2015;12:21.
Poulsen SS, Jackson P, Kling K, Knudsen KB, Skaug V, Kyjovska ZO, Thomsen BL, Clausen PA,
Atluri R, Berthing T, Bengtson S, Wolff H, Jensen KA, Wallin H, Vogel U. Multi-walled carbon
nanotube physicochemical properties predict pulmonary inflammation and genotoxicity.
Nanotoxicology 2016;10:1263-1275.
Poulsen SS, Jacobsen NR, Labib S, Wu D, Husain M, Williams A, Bøgelund JP, Andersen O,
Købler C, Mílhave K, Kyjovska ZO, Saber AT, Wallin H, Yauk CL, Vogel U, Halappanavar S.
Transcriptomic analysis reveals novel mechanistic insight into murine biological responses to
multi-walled carbon nanotubes in lungs and cultured lung epithelial cells. PLoS One
2013;8:e80452.
Poulsen SS, Knudsen KB, Jackson P, Weydahl IE, Saber AT, Wallin H, Vogel U. Multi-walled
carbon nanotube-physicochemical properties predict the systemic acute phase response following
pulmonary exposure in mice. PLoS One 2017;12:e0174167.
Poulsen SS, Saber AT, Mortensen A, Szarek J, Wu D, Williams A, Andersen O, Jacobsen NR, Yauk
CL, Wallin H, Halappanavar S, Vogel U. Changes in cholesterol homeostasis and acute phase
response link pulmonary exposure to multi-walled carbon nanotubes to risk of cardiovascular
disease. Toxicol Appl Pharmacol 2015a;283:210-222.
Poulsen SS, Saber AT, Williams A, Andersen O, Købler C, Atluri R, Pozzebon ME, Mucelli SP,
Simion M, Rickerby D, Mortensen A, Jackson P, Kyjovska ZO, Mílhave K, Jacobsen NR, Jensen
KA, Yauk CL, Wallin H, Halappanavar S, Vogel U. MWCNTs of different physicochemical
properties cause similar inflammatory responses, but differences in transcriptional and
histological markers of fibrosis in mouse lungs. Toxicol Appl Pharmacol 2015b;284.:16-32.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of
inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836-
843.
Rittinghausen S, Hackbarth A, Creutzenberg O, Ernst H, Heinrich U, Leonhardt A, Schaudien D.
The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after
intraperitoneal injection in rats. Part Fibre Toxicol 2014;11:59.
Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss
OR, Wong BA, Dodd DE, Andersen ME, Bonner JC. Inhaled carbon nanotubes reach the
subpleural tissue in mice. Nat Nanotechnol 2009;4:747-751.
55
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Saber AT, Jacobsen NR, Jackson P, Poulsen SS, Kyjovska ZO, Halappanavar S, Yauk CL, Wallin
H, Vogel U. Particle-induced pulmonary acute phase response may be the causal link between
particle inhalation and cardiovascular disease. Wiley Interdiscip Rev Nanomed Nanobiotechnol
2014;6:517-531.
Saber AT, Jacobsen NR, Mortensen A, Szarek J, Jackson P, Madsen AM, Jensen KA, Koponen IK,
Brunborg G, Gutzkow KB, Vogel U, Wallin H. Nanotitanium dioxide toxicity in mouse lung is
reduced in sanding dust from paint. Part Fibre Toxicol 2012a;9:4.
Saber AT, Jensen KA, Jacobsen NR, Birkedal R, Mikkelsen L, Míller P, Loft S, Wallin H, Vogel U.
Inflammatory and genotoxic effects of nanoparticles designed for inclusion in paints and lacquers.
Nanotoxicology 2012b;6:453-471.
Saber AT, Lamson JS, Jacobsen NR, Ravn-Haren G, Hougaard KS, Nyendi AN, Wahlberg P,
Madsen AM, Jackson P, Wallin H, Vogel U. Particle-induced pulmonary acute phase response
correlates with neutrophil influx linking inhaled particles and cardiovascular risk. PLoS One
2013;8:e69020.
Saber AT, Mortensen A, Szarek J, Koponen IK, Levin M, Jacobsen NR, Pozzebon ME, Mucelli SP,
Rickerby DG, Kling K, Atluri R, Madsen AM, Jackson P, Kyjovska ZO, Vogel U, Jensen KA,
Wallin H. Epoxy composite dusts with and without carbon nanotubes cause similar pulmonary
responses, but differences in liver histology in mice following pulmonary deposition. Part Fibre
Toxicol 2016;13:37.
Sager TM, Wolfarth MW, Andrew M, Hubbs A, Friend S, Chen TH, Porter DW, Wu N, Yang F,
Hamilton RF, Holian A. Effect of multi-walled carbon nanotube surface modification on
bioactivity in the C57BL/6 mouse model. Nanotoxicology 2014;8:317-327.
Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, Kashon ML,
Mercer RR, Bauer AK, Chen BT, Salisbury JL, Frazer D, McKinney W, Andrew M, Tsuruoka S,
Endo M, Fluharty KL, Castranova V, Reynolds SH. Promotion of lung adenocarcinoma following
inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol 2014;11:3.
Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Doyle CD, West JL,
Billups WE, Ausman KD, Colvin VL. Functionalization density dependence of single-walled
carbon nanotubes cytotoxicity in vitro. Toxicol Lett 2006;161:135-142.
SCHER/SCCP/SCENIHR. Risk assessment methodologies and approaches for genotoxic and
carcinogenic substances. European Commission Health & Consumer Protection, 2009.
http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_113.pdf
Schmid O, Cassee FR. On the pivotal role of dose for particle toxicology and risk assessment:
Exposure is a poor surrogate for delivered dose. Part Fibre Toxicol 2017;14:52.
Shvedova AA, Yanamala N, Kisin ER, Khailullin TO, Birch ME, Fatkhutdinova LM. Integrated
analysis of dysregulated ncRNA and mRNA expression profiles in humans exposed to carbon
nanotubes. PLoS One 2016;11:e0150628.
Steinmetz A, Hocke G, Saile R, Puchois P, Fruchart JC. Influence of serum amyloid A on
cholesterol esterification in human plasma. Biochim Biophys Acta 1989;1006:173-178.
56
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Suzui M, Futakuchi M, Fukamachi K, Numano T, Abdelgied M, Takahashi S, Ohnishi M, Omori
T, Tsuruoka S, Hirose A, Kanno J, Sakamoto Y, Alexander DB, Alexander WT, Jiegou X, Tsuda H.
Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of
pleural malignant mesothelioma and lung tumors. Cancer Sci 2016;107:924-935.
Suzuki Y, Tada-Oikawa S, Hayashi Y, Izuoka K, Kataoka M, Ichikawa S, Wu W, Zong C, Ichihara
G, Ichihara S. Single- and double-walled carbon nanotubes enhance atherosclerogenesis by
promoting monocyte adhesion to endothelial cells and endothelial progenitor cell dysfunction.
Part Fibre Toxicol 2016;13:54.
Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose-dependent mesothelioma induction by
intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer
Sci 2012;103:1440-1444.
Takaya M, Ono-Ogasawara M, Shinohara Y, Kubota H, Tsuruoka S, Koda S. Evaluation of
exposure risk in the weaving process of MWCNT-coated yarn with real-time particle
concentration measurements and characterization of dust particles. Ind Health 2012;50:147-155.
Thompson JC, Wilson PG, Shridas P, Ji A, de BM, de Beer FC, Webb NR, Tannock LR. Serum
amyloid A3 is pro-atherogenic. Atherosclerosis 2018;268:32-35.
Umeda Y, Kasai T, Saito M, Kondo H, Toya T, Aiso S, Okuda H, Nishizawa T, Fukushima S. Two-
week toxicity of multi-walled carbon nanotubes by whole-body inhalation exposure in rats. J
Toxicol Pathol 2013;26:131-140.
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J.
Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of
intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054-2061.
Waris G, Ahsan H. Reactive oxygen species: Role in the development of cancer and various
chronic conditions. J Carcinog 2006;5:14.
Whitehead AS, Zahedi K, Rits M, Mortensen RF, Lelias JM. Mouse C-reactive protein. Generation
of cDNA clones, structural analysis, and induction of mRNA during inflammation. Biochem J
1990;266:283-290.
World Health Organization. Cardiovascular Diseases (CVDs). World Health Organization, 2018.
http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
Yamashita K, Yoshioka Y, Higashisaka K, Morishita Y, Yoshida T, Fujimura M, Kayamuro H,
Nabeshi H, Yamashita T, Nagano K, Abe Y, Kamada H, Kawai Y, Mayumi T, Yoshikawa T, Itoh
N, Tsunoda S, Tsutsumi Y. Carbon nanotubes elicit DNA damage and inflammatory response
relative to their size and shape. Inflammation 2010;33:276-280.
Zhang M, Li J. Carbon nanotube in different shapes. Materials today 2006;12:12-18.
57
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
BEU, Alm.del - 2019-20 - Bilag 101: Orientering om NFA’s forslag til grænseværdier for fem kemiske stoffer, fra beskæftigelsesministeren
Lersø Parkallé 105
DK-2100 Copenhagen
T +45 3916 5200
F +45 3916 5201
E nfa@nfa.dk
W www.nfa.dk