Bacillus
amyloliquefaciens strain
FZB24
Bacillus
amyloliquefaciens strain
MBI 600
Beauveria bassiana
strain 147
Beauveria bassiana
strain NPP111B005
Clayed charcoal
Cyclaniliprole
SANTE/10656/2017 Rev. 1
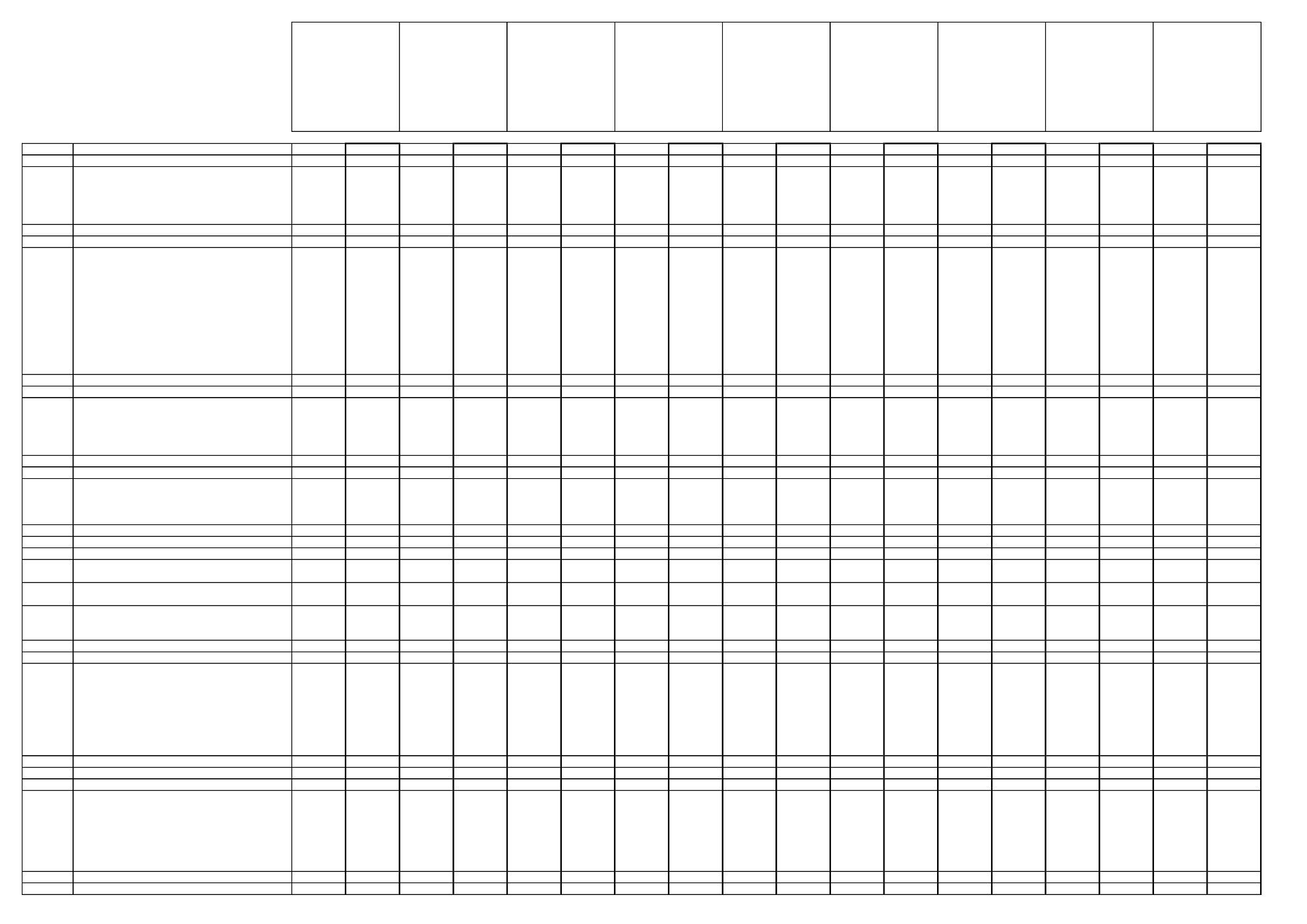
code
0100000
0110000
0110010
0110020
0110030
0110040
0110050
0110990
0120000
0120010
0120020
0120030
0120040
0120050
0120060
0120070
0120080
0120090
0120100
0120110
0120990
0130000
0130010
0130020
0130030
0130040
0130050
0130990
0140000
0140010
0140020
0140030
0140040
0140990
0150000
0151000
0151010
0151020
0152000
0153000
0153010
0153020
0153030
0153990
0154000
0154010
0154020
0154030
0154040
0154050
0154060
0154070
0154080
0154990
0160000
0161000
0161010
0161020
0161030
0161040
0161050
0161060
0161070
0161990
0162000
Commodities
FRUITS, FRESH or FROZEN; TREE NUTS
Citrus fruits
Grapefruits
Oranges
Lemons
Limes
Mandarins
Others
Tree nuts
Almonds
Brazil nuts
Cashew nuts
Chestnuts
Coconuts
Hazelnuts/cobnuts
Macadamias
Pecans
Pine nut kernels
Pistachios
Walnuts
Others
Pome fruits
Apples
Pears
Quinces
Medlars
Loquats/Japanese medlars
Others
Stone fruits
Apricots
Cherries (sweet)
Peaches
Plums
Others
Berries and small fruits
(a) grapes
Table grapes
Wine grapes
(b) strawberries
(c) cane fruits
Blackberries
Dewberries
Raspberries (red and yellow)
Others
(d) other small fruits and berries
Blueberries
Cranberries
Currants (black, red and white)
Gooseberries (green, red and yellow)
Rose hips
Mulberries (black and white)
Azaroles/Mediterranean medlars
Elderberries
Others
Miscellaneous fruits with
(a) edible peel
Dates
Figs
Table olives
Kumquats
Carambolas
Kaki/Japanese persimmons
Jambuls/jambolans
Others
(b) inedible peel, small
Current
New
Current
New
Current
New
Current
New
Current
New
Current
New
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
0.01*
Dichlorprop (Sum of
Ethephon
dichlorprop (including
dichlorprop-P), its salts,
esters and conjugates,
expressed as dichlorprop
(R)
Etridiazole
Current
New
0.3
0.3
0.3
0.3
0.3
0.3
0.3
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
Current
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.1
0.1
0.1
0.1
0.1
0.2
0.1
0.1
0.1
0.1
0.5
0.1
0.8
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
5
0.05*
0.05*
0.05*
New
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.1
0.1
0.1
0.1
0.1
0.2
0.1
0.1
0.1
0.1
0.5
0.1
0.8
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
5
0.05*
0.05*
0.05*
Current
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.1
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
New
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.1
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.02*
0.3
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
0.02*
1
2
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
20
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
1
2
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
20
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
3
7
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
0.05*
3
7
0.05*
0.05*
0.3
0.05*
0.05*
0.05*