Sundheds- og Forebyggelsesudvalget 2012-13
SUU Alm.del Bilag 212
Offentligt
Cytotec�misoprostol tablets
WARNINGSCYTOTEC (MISOPROSTOL) ADMINISTRATION TO WOMEN WHO AREPREGNANT CAN CAUSE BIRTH DEFECTS, ABORTION, OR PREMATUREBIRTH. UTERINE RUPTURE HAS BEEN REPORTED WHEN CYTOTEC WASADMINISTERED IN PREGNANT WOMEN TO INDUCE LABOR OR TO INDUCEABORTION BEYOND THE EIGHTH WEEK OF PREGNANCY (see alsoPRECAUTIONSandLABOR AND DELIVERY).CYTOTEC SHOULD NOT BETAKEN BY PREGNANT WOMEN TO REDUCE THE RISK OF ULCERS INDUCEDBY NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDs) (seeCONTRAINDICATIONS, WARNINGS,andPRECAUTIONS).PATIENTS MUST BE ADVISED OF THE ABORTIFACIENT PROPERTY ANDWARNED NOT TO GIVE THE DRUG TO OTHERS.Cytotec should not be used for reducing the risk of NSAID-induced ulcers in women ofchildbearing potential unless the patient is at high risk of complications from gastriculcers associated with use of the NSAID, or is at high risk of developing gastriculceration. In such patients, Cytotec may be prescribed if the patient• has had a negative serum pregnancy test within 2 weeks prior to beginning therapy.• is capable of complying with effective contraceptive measures.• has received both oral and written warnings of the hazards of misoprostol, the risk ofpossible contraception failure, and the danger to other women of childbearingpotential should the drug be taken by mistake.• will begin Cytotec only on the second or third day of the next normal menstrualperiod.
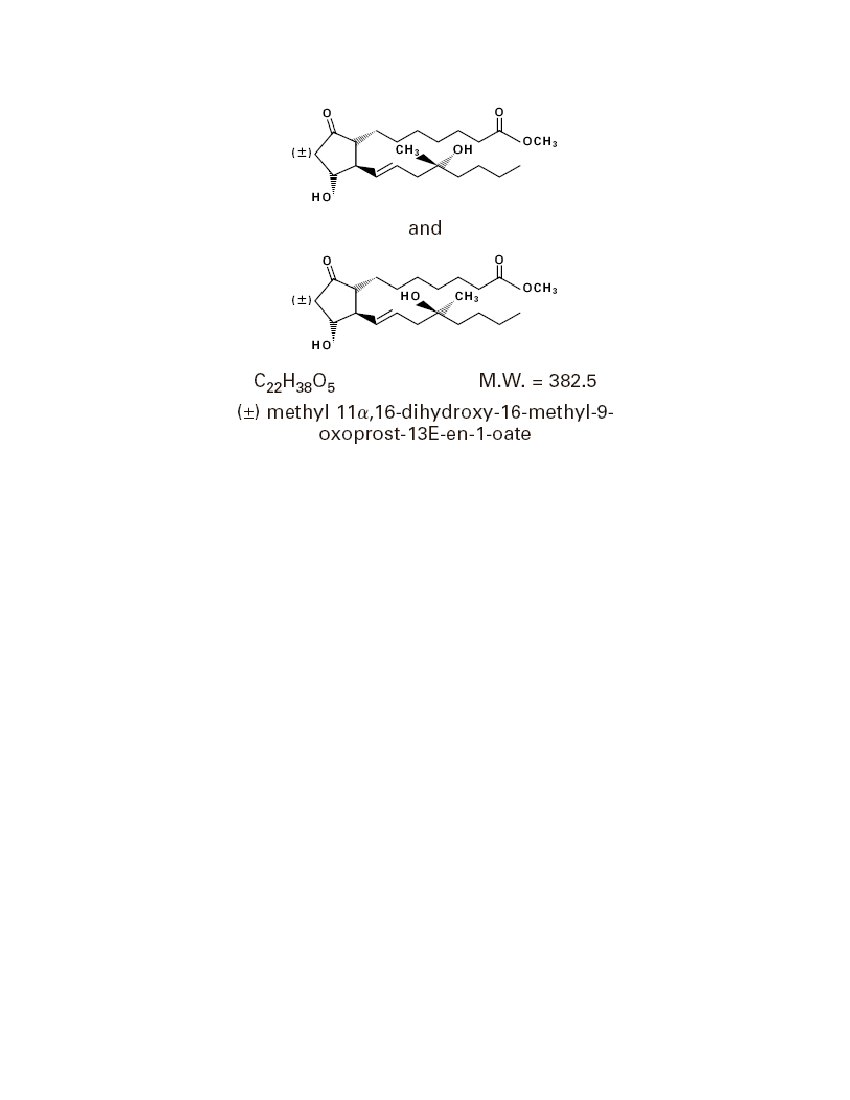
DESCRIPTIONCytotec oral tablets contain either 100 mcg or 200 mcg of misoprostol, a syntheticprostaglandin E1analog.Misoprostol contains approximately equal amounts of the two diastereomers presentedbelow with their enantiomers indicated by (�):
1
Reference ID: 3217917
Misoprostol is a water-soluble, viscous liquid.Inactive ingredients of tablets are hydrogenated castor oil, hypromellose, microcrystallinecellulose, and sodium starch glycolate.CLINICAL PHARMACOLOGYPharmacokinetics:Misoprostol is extensively absorbed, and undergoes rapid de·esterification to its free acid, which is responsible for its clinical activity and, unlike theparent compound, is detectable in plasma. The alpha side chain undergoes beta oxidationand the beta side chain undergoes omega oxidation followed by reduction of the ketone togive prostaglandin F analogs.In normal volunteers, Cytotec (misoprostol) is rapidly absorbed after oral administrationwith a Tmaxof misoprostol acid of 12 � 3 minutes and a terminal half-life of 20–40minutes.There is high variability of plasma levels of misoprostol acid between and within studiesbut mean values after single doses show a linear relationship with dose over the range of200–400 mcg. No accumulation of misoprostol acid was noted in multiple dose studies;plasma steady state was achieved within two days.Maximum plasma concentrations of misoprostol acid are diminished when the dose istaken with food and total availability of misoprostol acid is reduced by use ofconcomitant antacid. Clinical trials were conducted with concomitant antacid, however,so this effect does not appear to be clinically important.
2
Reference ID: 3217917
Mean � SDFastingWith AntacidWith High Fat...Breakfast*
Cmax(pg/ml)811 � 317689 � 315303 � 176*
AUC(0–4)(pg¶hr/ml)417 � 135349 � 108*373 � 111
Tmax(min)14 � 820 � 1464 � 79*
Comparisons with fasting results statistically significant, p<0.05.
After oral administration of radiolabeled misoprostol, about 80% of detected radioactivityappears in urine. Pharmacokinetic studies in patients with varying degrees of renalimpairment showed an approximate doubling of T1/2, Cmax, and AUC compared tonormals, but no clear correlation between the degree of impairment and AUC. In subjectsover 64 years of age, the AUC for misoprostol acid is increased. No routine dosageadjustment is recommended in older patients or patients with renal impairment, butdosage may need to be reduced if the usual dose is not tolerated.Drug interaction studies between misoprostol and several nonsteroidal anti-inflammatorydrugs showed no effect on the kinetics of ibuprofen or diclofenac, and a 20% decrease inaspirin AUC, not thought to be clinically significant.Pharmacokinetic studies also showed a lack of drug interaction with antipyrine andpropranolol when these drugs were given with misoprostol. Misoprostol given for 1 weekhad no effect on the steady state pharmacokinetics of diazepam when the two drugs wereadministered 2 hours apart.The serum protein binding of misoprostol acid is less than 90% and is concentration-independent in the therapeutic range.After a single oral dose of misoprostol to nursing mothers, misoprostol acid was excretedin breast milk. The maximum concentration of misoprostol acid in expressed breast milkwas achieved within 1 hour after dosing and was 7.6 pg/ml (CV 37%) and 20.9 pg/ml(CV 62%) after single 200�gand 600�gmisoprostol administration, respectively. Themisoprostol acid concentrations in breast milk declined to < 1 pg/ml at 5 hours post-dose.Pharmacodynamics:Misoprostol has both antisecretory (inhibiting gastric acidsecretion) and (in animals) mucosal protective properties. NSAIDs inhibit prostaglandinsynthesis, and a deficiency of prostaglandins within the gastric mucosa may lead todiminishing bicarbonate and mucus secretion and may contribute to the mucosal damagecaused by these agents. Misoprostol can increase bicarbonate and mucus production, butin man this has been shown at doses 200 mcg and above that are also antisecretory. It istherefore not possible to tell whether the ability of misoprostol to reduce the risk ofgastric ulcer is the result of its antisecretory effect, its mucosal protective effect, or both.In vitrostudies on canine parietal cells using tritiated misoprostol acid as the ligand haveled to the identification and characterization of specific prostaglandin receptors. Receptorbinding is saturable, reversible, and stereospecific. The sites have a high affinity for3
Reference ID: 3217917
misoprostol, for its acid metabolite, and for other E type prostaglandins, but not for F or Iprostaglandins and other unrelated compounds, such as histamine or cimetidine.Receptor-site affinity for misoprostol correlates well with an indirect index ofantisecretory activity. It is likely that these specific receptors allow misoprostol takenwith food to be effective topically, despite the lower serum concentrations attained.Misoprostol produces a moderate decrease in pepsin concentration during basalconditions, but not during histamine stimulation. It has no significant effect on fasting orpostprandial gastrin nor on intrinsic factor output.Effects on gastric acid secretion:Misoprostol, over the range of 50–200 mcg,inhibits basal and nocturnal gastric acid secretion, and acid secretion in response to avariety of stimuli, including meals, histamine, pentagastrin, and coffee. Activity isapparent 30 minutes after oral administration and persists for at least 3 hours. In general,the effects of 50 mcg were modest and shorter lived, and only the 200-mcg dose hadsubstantial effects on nocturnal secretion or on histamine and meal-stimulated secretion.Uterine effects:Cytotec has been shown to produce uterine contractions that mayendanger pregnancy. (See boxedWARNINGS.)Other pharmacologic effects:Cytotec does not produce clinically significant effectson serum levels of prolactin, gonadotropins, thyroid-stimulating hormone, growthhormone, thyroxine, cortisol, gastrointestinal hormones (somatostatin, gastrin, vasoactiveintestinal polypeptide, and motilin), creatinine, or uric acid. Gastric emptying,immunologic competence, platelet aggregation, pulmonary function, or thecardiovascular system are not modified by recommended doses of Cytotec.Clinical studies:In a series of small short-term (about 1 week) placebo-controlledstudies in healthy human volunteers, doses of misoprostol were evaluated for their abilityto reduce the risk of NSAID-induced mucosal injury. Studies of 200 mcg q.i.d. ofmisoprostol with tolmetin and naproxen, and of 100 and 200 mcg q.i.d. with ibuprofen,all showed reduction of the rate of significant endoscopic injury from about 70–75% onplacebo to 10–30% on misoprostol. Doses of 25–200 mcg q.i.d. reduced aspirin-inducedmucosal injury and bleeding.Reducing the risk of gastric ulcers caused by nonsteroidalanti-inflammatory drugs (NSAIDs):Two 12-week, randomized, double-blind trialsin osteoarthritic patients who had gastrointestinal symptoms but no ulcer on endoscopywhile taking an NSAID compared the ability of 200 mcg of Cytotec, 100 mcg of Cytotec,and placebo to reduce the risk of gastric ulcer (GU) formation. Patients wereapproximately equally divided between ibuprofen, piroxicam, and naproxen, andcontinued this treatment throughout the 12 weeks. The 200-mcg dose caused a marked,statistically significant reduction in gastric ulcers in both studies. The lower dose wassomewhat less effective, with a significant result in only one of the studies.
4
Reference ID: 3217917
TherapyStudy No. 1Cytotec 200 mcgq.i.d. (n=74)Cytotec 100 mcgq.i.d. (n=77)Placebo (n=76)Study No. 2Cytotec 200 mcgq.i.d. (n=65)Cytotec 100 mcgq.i.d. (n=66)Placebo (n=62)Studies No. 1 & No. 2**Cytotec 200 mcgq.i.d. (n=139)Cytotec 100 mcgq.i.d. (n=143)Placebo (n=138)***
Reduction of Risk of Gastric Ulcers Induced by
Ibuprofen, Piroxicam, or Naproxen
[No. of patients with ulcer(s) (%)]Therapy Duration4 weeks8 weeks12 weeks1 (1.4)3 (3.9)11 (14.5)1 (1.5)2 (3.0)6 (9.7)2 (1.4)5 (3.5)17 (12.3)0........1 (1.3)4 (5.3)1 (1.5)2 (3.0)2 (3.2)1 (0.7)3 (2.1)6 (4.3)0........1 (1.3)4 (5.3)0........1 (1.5)3 (4.8)0........2 (1.4)7 (5.1)1 (1.4)*5 (6.5)*19 (25.0)2 (3.1)*5 (7.6)11 (17.7)3 (2.2)*10 (7.0)*30 (21.7)
Statistically significantly different from placebo at the 5% level.Combined data from Study No. 1 and Study No. 2.
In these trials there were no significant differences between Cytotec and placebo in reliefof day or night abdominal pain. No effect of Cytotec in reducing the risk of duodenalulcers was demonstrated, but relatively few duodenal lesions were seen.In another clinical trial, 239 patients receiving aspirin 650–1300 mg q.i.d. for rheumatoidarthritis who had endoscopic evidence of duodenal and/or gastric inflammation wererandomized to misoprostol 200 mcg q.i.d. or placebo for 8 weeks while continuing toreceive aspirin. The study evaluated the possible interference of Cytotec on the efficacyof aspirin in these patients with rheumatoid arthritis by analyzing joint tenderness, jointswelling, physician’s clinical assessment, patient’s assessment, change in ARAclassification, change in handgrip strength, change in duration of morning stiffness,patient’s assessment of pain at rest, movement, interference with daily activity, and ESR.Cytotec did not interfere with the efficacy of aspirin in these patients with rheumatoidarthritis.INDICATIONS AND USAGECytotec (misoprostol) is indicated for reducing the risk of NSAID (nonsteroidal anti-inflammatory drugs, including aspirin)–induced gastric ulcers in patients at high risk ofcomplications from gastric ulcer, e.g., the elderly and patients with concomitantdebilitating disease, as well as patients at high risk of developing gastric ulceration, such5Reference ID: 3217917
as patients with a history of ulcer. Cytotec has not been shown to reduce the risk ofduodenal ulcers in patients taking NSAIDs. Cytotec should be taken for the duration ofNSAID therapy. Cytotec has been shown to reduce the risk of gastric ulcers in controlledstudies of 3 months’ duration. It had no effect, compared to placebo, on gastrointestinalpain or discomfort associated with NSAID use.CONTRAINDICATIONSSee boxedWARNINGS.Cytotec should not be taken by pregnant women to reduce the risk of ulcers inducedby nonsteroidal anti-inflammatory drugs (NSAIDs).Cytotec should not be taken by anyone with a history of allergy to prostaglandins.WARNINGSSee boxedWARNINGS.For hospital use only if misoprostol were to be used for cervical ripening, induction oflabor, or for the treatment of serious post-partum hemorrhage, which are outside of theapproved indication.PRECAUTIONSCaution should be employed when administering Cytotec (misoprostol) to patients withpre-existing cardiovascular disease.Information for patients:Women of childbearing potential using Cytotec to decreasethe risk of NSAID-induced ulcers should be told that they must not be pregnant whenCytotec therapy is initiated, and that they must use an effective contraception methodwhile taking Cytotec.See boxedWARNINGS.Cytotec is intended for administration along with nonsteroidal anti-inflammatory drugs(NSAIDs), including aspirin, to decrease the chance of developing an NSAID-inducedgastric ulcer.Cytotec should be taken only according to the directions given by a physician.If the patient has questions about or problems with Cytotec, the physician should becontacted promptly.THE PATIENT SHOULD NOT GIVE CYTOTEC TO ANYONE ELSE.Cytotec hasbeen prescribed for the patient’s specific condition, may not be the correct treatment foranother person, and may be dangerous to the other person if she were to becomepregnant.
6
Reference ID: 3217917
The Cytotec package the patient receives from the pharmacist will include a leafletcontaining patient information. The patient should read the leaflet before taking Cytotecand each time the prescription is renewed because the leaflet may have been revised.Keep Cytotec out of the reach of children.SPECIAL NOTE FOR WOMEN: Cytotec may cause birth defects, abortion(sometimes incomplete), or premature labor if given to pregnant women.Cytotec is available only as a unit-of-use package that includes a leaflet containingpatient information. SeePatient Informationat the end of this labeling.Drug interactions:SeeClinical Pharmacology.Cytotec has not been shown tointerfere with the beneficial effects of aspirin on signs and symptoms of rheumatoidarthritis. Cytotec does not exert clinically significant effects on the absorption, bloodlevels, and antiplatelet effects of therapeutic doses of aspirin. Cytotec has no clinicallysignificant effect on the kinetics of diclofenac or ibuprofen.Prostaglandins such as Cytotec may augment the activity of oxytocic agents, especiallywhen given less than 4 hours prior to initiating oxytocin treatment. Concomitant use isnot recommended.Animal toxicology:A reversible increase in the number of normal surface gastricepithelial cells occurred in the dog, rat, and mouse. No such increase has been observedin humans administered Cytotec for up to 1 year.An apparent response of the female mouse to Cytotec in long-term studies at 100 to 1000times the human dose was hyperostosis, mainly of the medulla of sternebrae.Hyperostosis did not occur in long-term studies in the dog and rat and has not been seenin humans treated with Cytotec.Carcinogenesis, mutagenesis, impairment of fertility:There was no evidence ofan effect of Cytotec on tumor occurrence or incidence in rats receiving daily doses up to150 times the human dose for 24 months. Similarly, there was no effect of Cytotec ontumor occurrence or incidence in mice receiving daily doses up to 1000 times the humandose for 21 months. The mutagenic potential of Cytotec was tested in severalin vitroassays, all of which were negative.Misoprostol, when administered to breeding male and female rats at doses 6.25 times to625 times the maximum recommended human therapeutic dose, produced dose-relatedpre- and post-implantation losses and a significant decrease in the number of live pupsborn at the highest dose. These findings suggest the possibility of a general adverse effecton fertility in males and females.
7
Reference ID: 3217917
Pregnancy: Pregnancy Category X.Teratogenic effects:See boxedWARNINGS.Congenital anomalies sometimesassociated with fetal death have been reported subsequent to the unsuccessful use ofmisoprostol as an abortifacient, but the drug's teratogenic mechanism has not beendemonstrated. Several reports in the literature associate the use of misoprostol during thefirst trimester of pregnancy with skull defects, cranial nerve palsies, facial malformations,and limb defects.Cytotec is not fetotoxic or teratogenic in rats and rabbits at doses 625 and 63 times thehuman dose, respectively.Nonteratogenic effects:See boxedWARNINGS.Cytotec may endanger pregnancy(may cause abortion) and thereby cause harm to the fetus when administered to apregnant woman. Cytotec may produce uterine contractions, uterine bleeding, andexpulsion of the products of conception. Abortions caused by Cytotec may beincomplete. If a woman is or becomes pregnant while taking this drug to reduce the riskof NSAID-induced ulcers, the drug should be discontinued and the patient apprised of thepotential hazard to the fetus.Labor and delivery:Cytotec can induce or augment uterine contractions. Vaginaladministration of Cytotec, outside of its approved indication, has been used as a cervicalripening agent, for the induction of labor and for treatment of serious postpartumhemorrhage in the presence of uterine atony. A major adverse effect of the obstetrical useof Cytotec is uterine tachysystole which may progress to uterine tetany with markedimpairment of uteroplacental blood flow, uterine rupture (requiring surgical repair,hysterectomy, and/or salpingo-oophorectomy), or amniotic fluid embolism and lead toadverse fetal heart changes. Uterine activity and fetal status should be monitored bytrained obstetrical personnel in a hospital setting.The risk of uterine rupture increases with advancing gestational ages and prior uterinesurgery, including Cesarean delivery. Grand multiparity also appears to be a risk factorfor uterine rupture.The use of Cytotec outside of its approved indication may also be associated withmeconium passage, meconium staining of amniotic fluid, and Cesarean delivery.Maternal shock, maternal death, fetal bradycardia, and fetal death have also been reportedwith the use of misoprostol.Cytotec should not be used in the third trimester in women with a history of Cesareansection or major uterine surgery because of an increased risk of uterine rupture. Cytotecshould not be used in cases where uterotonic drugs are generally contraindicated or wherehyperstimulation of the uterus is considered inappropriate, such as cephalopelvicdisproportion, grand multiparity, hypertonic or hyperactive uterine patterns, or fetaldistress where delivery is not imminent, or when surgical intervention is moreappropriate.
8
Reference ID: 3217917
The effect of Cytotec on later growth, development, and functional maturation of thechild when Cytotec is used for cervical ripening or induction of labor has not beenestablished. Information on Cytotec's effect on the need for forceps delivery or otherintervention is unknown.Nursing mothers:Misoprostol is rapidly metabolized in the mother to misoprostolacid, which is biologically active and is excreted in breast milk. There are no publishedreports of adverse effects of misoprostol in breast-feeding infants of mothers takingmisoprostol. Caution should be exercised when misoprostol is administered to a nursingwoman.Pediatric use:Safety and effectiveness of Cytotec in pediatric patients have not beenestablished.ADVERSE REACTIONSThe following have been reported as adverse events in subjects receiving Cytotec:Gastrointestinal:In subjects receiving Cytotec 400 or 800 mcg daily in clinical trials,the most frequent gastrointestinal adverse events were diarrhea and abdominal pain. Theincidence of diarrhea at 800 mcg in controlled trials in patients on NSAIDs ranged from14–40% and in all studies (over 5,000 patients) averaged 13%. Abdominal pain occurredin 13–20% of patients in NSAID trials and about 7% in all studies, but there was noconsistent difference from placebo.Diarrhea was dose related and usually developed early in the course of therapy (after 13days), usually was self-limiting (often resolving after 8 days), but sometimes requireddiscontinuation of Cytotec (2% of the patients). Rare instances of profound diarrhealeading to severe dehydration have been reported. Patients with an underlying conditionsuch as inflammatory bowel disease, or those in whom dehydration, were it to occur,would be dangerous, should be monitored carefully if Cytotec is prescribed. Theincidence of diarrhea can be minimized by administering after meals and at bedtime, andby avoiding coadministration of Cytotec with magnesium-containing antacids.Gynecological:Women who received Cytotec during clinical trials reported thefollowing gynecological disorders: spotting (0.7%), cramps (0.6%), hypermenorrhea(0.5%), menstrual disorder (0.3%) and dysmenorrhea (0.1%). Postmenopausal vaginalbleeding may be related to Cytotec administration. If it occurs, diagnostic workup shouldbe undertaken to rule out gynecological pathology. (See boxedWARNINGS.)Elderly:There were no significant differences in the safety profile of Cytotec inapproximately 500 ulcer patients who were 65 years of age or older compared withyounger patients.Additional adverse events which were reported are categorized as follows:
9
Reference ID: 3217917
Incidence greater than 1%:In clinical trials, the following adverse reactions werereported by more than 1% of the subjects receiving Cytotec and may be causally relatedto the drug: nausea (3.2%), flatulence (2.9%), headache (2.4%), dyspepsia (2.0%),vomiting (1.3%), and constipation (1.1%). However, there were no significant differencesbetween the incidences of these events for Cytotec and placebo.Causal relationship unknown:The following adverse events were infrequentlyreported. Causal relationships between Cytotec and these events have not beenestablished but cannot be excluded:Body as a whole:aches/pains, asthenia, fatigue, fever, chills, rigors, weight changes.Skin:rash, dermatitis, alopecia, pallor, breast pain.Special senses:abnormal taste, abnormal vision, conjunctivitis, deafness, tinnitus,earache.Respiratory:upper respiratory tract infection, bronchitis, bronchospasm, dyspnea,pneumonia, epistaxis.Cardiovascular:chest pain, edema, diaphoresis, hypotension, hypertension, arrhythmia,phlebitis, increased cardiac enzymes, syncope, myocardial infarction (some fatal),thromboembolic events (e.g., pulmonary embolism, arterial thrombosis, and CVA).Gastrointestinal:GI bleeding, GI inflammation/infection, rectal disorder, abnormalhepatobiliary function, gingivitis, reflux, dysphagia, amylase increase.Hypersensitivity:anaphylactic reactionMetabolic:glycosuria, gout, increased nitrogen, increased alkaline phosphatase.Genitourinary:polyuria, dysuria, hematuria, urinary tract infection.Nervous system/Psychiatric:anxiety, change in appetite, depression, drowsiness,dizziness, thirst, impotence, loss of libido, sweating increase, neuropathy, neurosis,confusion.Musculoskeletal:arthralgia, myalgia, muscle cramps, stiffness, back pain.Blood/Coagulation:anemia, abnormal differential, thrombocytopenia, purpura, ESRincreased.OVERDOSAGEThe toxic dose of Cytotec in humans has not been determined. Cumulative total dailydoses of 1600 mcg have been tolerated, with only symptoms of gastrointestinaldiscomfort being reported. In animals, the acute toxic effects are diarrhea, gastrointestinal10
Reference ID: 3217917
lesions, focal cardiac necrosis, hepatic necrosis, renal tubular necrosis, testicular atrophy,respiratory difficulties, and depression of the central nervous system. Clinical signs thatmay indicate an overdose are sedation, tremor, convulsions, dyspnea, abdominal pain,diarrhea, fever, palpitations, hypotension, or bradycardia. Symptoms should be treatedwith supportive therapy.It is not known if misoprostol acid is dialyzable. However, because misoprostol ismetabolized like a fatty acid, it is unlikely that dialysis would be appropriate treatmentfor overdosage.DOSAGE AND ADMINISTRATIONThe recommended adult oral dose of Cytotec for reducing the risk of NSAID-inducedgastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, adose of 100 mcg can be used. (SeeClinical Pharmacology: Clinical studies.)Cytotecshould be taken for the duration of NSAID therapy as prescribed by the physician.Cytotec should be taken with a meal, and the last dose of the day should be at bedtime.Renal impairment:Adjustment of the dosing schedule in renally impaired patients isnot routinely needed, but dosage can be reduced if the 200-mcg dose is not tolerated. (SeeClinical Pharmacology.)HOW SUPPLIEDCytotec 100-mcg tablets are white, round, with SEARLE debossed on one side and 1451on the other side; supplied as:NDC Number0025-1451-600025-1451-200025-1451-34Sizeunit-of-use bottle of 60unit-of-use bottle of 120carton of 100 unit dose
Cytotec 200-mcg tablets are white, hexagonal, with SEARLE debossed above and 1461debossed below the line on one side and a double stomach debossed on the other side;supplied as:NDC Number0025-1461-600025-1461-310025-1461-34Sizeunit-of-use bottle of 60unit-of-use bottle of 100carton of 100 unit dose
Store at or below 25�C (77�F), in a dry area.This product’s label may have been updated. For current full prescribing information,please visit www.pfizer.com.
11
Reference ID: 3217917
LAB-0170-3.1Revised November 2012
12Reference ID: 3217917
PATIENT INFORMATION
Read this leaflet before taking Cytotec�(misoprostol) and each time your prescription isrenewed, because the leaflet may be changed.Cytotec (misoprostol) is being prescribed by your doctor to decrease the chance ofgetting stomach ulcers related to the arthritis/pain medication that you take.Do not take Cytotec to reduce the risk of NSAID-induced ulcers if you are pregnant. (SeeboxedWARNINGS.)Cytotec can cause abortion (sometimes incomplete which couldlead to dangerous bleeding and require hospitalization and surgery), premature birth, orbirth defects. It is also important to avoid pregnancy while taking this medication and forat least one month or through one menstrual cycle after you stop taking it. Cytotec hasbeen reported to cause the uterus to rupture (tear) when given after the eighth week ofpregnancy. Rupture (tearing) of the uterus can result in severe bleeding, hysterectomy,and/or maternal or fetal death.If you become pregnant during Cytotec therapy, stop taking Cytotec and contact yourphysician immediately. Remember that even if you are on a means of birth control it isstill possible to become pregnant. Should this occur, stop taking Cytotec and contact yourphysician immediately.Cytotec may cause diarrhea, abdominal cramping, and/or nausea in some people. In mostcases these problems develop during the first few weeks of therapy and stop after about aweek. You can minimize possible diarrhea by making sure you take Cytotec with food.Because these side effects are usually mild to moderate and usually go away in a matterof days, most patients can continue to take Cytotec. If you have prolonged difficulty(more than 8 days), or if you have severe diarrhea, cramping and/or nausea, call yourdoctor.Take Cytotec only according to the directions given by your physician.Do not give Cytotec to anyone else. It has been prescribed for your specific condition,may not be the correct treatment for another person, and would be dangerous if the otherperson were pregnant.This information sheet does not cover all possible side effects of Cytotec. This patientinformation leaflet does not address the side effects of your arthritis/pain medication. Seeyour doctor if you have questions.Keep out of reach of children.This product’s label may have been updated. For current full prescribing information,please visit www.pfizer.com.
13
Reference ID: 3217917
LAB-0172–0.1Revised September 2012
14Reference ID: 3217917