Miljøudvalget 2012-13
MIU Alm.del Bilag 262
Offentligt
1
Input for the REACH-review in 2013 on endocrinedisrupters(tærskelværdi-projekt, j.nr. MST-621-00050)Final report 21 March 2013DANISH CENTRE ON ENDOCRINE DISRUPTERSUlla Hass, Sofie Christiansen, Marta AxelstadKarin Dreisig Sørensen and Julie BobergDivision of Toxicology and Risk Assessment, National Food Institute,Technical University of Denmark
2
Contents1. Terms of reference and scope2. Background and aim3. Threshold for EDs3.1 What is a threshold?3.2 Biological thresholds for EDs?3.3 Do toxicological data indicate threshold or no-threshold?3.4 Conclusions4. Non-monotonic dose-response (NMDR) for EDs?4.1 ED mechanisms for NMDR4.2 Human evidence4.3 NMDRin vitro4.3.1 Critical review ofin vitrostudies included in Vandenberg et al. 20124.4 NMDRin vivo4.4.1 Critical review ofin vivostudies included in Vandenberg et al. 20124.4.1.1 NMDR and reproductive organ weights4.4.1.2 NMDR and timing of puberty and nipple retention in male offspring4.5 Conclusions5. Uncertainties related to regulatory requirements and test methods5.1 Current REACH information requirements, test methods5.1.1 Repeated dose toxicity5.1.2 Carcinogenicity5.1.3 Reproductive toxicity5.1.4 Sensitivity for finding a relevant threshold-like dose for EDs, using poweranalysis5.2 Conclusions6. Are EDs of particular concern?7. Summary, conclusions and recommendations8. ReferencesAppendix 1 Details on the evaluatedin vitrostudiesAppendix 2 Details on the evaluatedin vivostudiesAppendix 3 Sensitivity for finding threshold-like doses, based on power analysis33455791010121213151517182121212222232425262731353948
3
1. Terms of reference and scopeThis report has been prepared by the Danish Centre on Endocrine Disrupters (CeHoS) as a projectcontracted by the Danish Environmental Protection Agency. The Danish Centre on EndocrineDisrupters is an interdisciplinary scientific network without walls. The main purpose of the Centreis to build and gather new knowledge on endocrine disrupters with the focus on providinginformation requested for the preventive work of the regulatory authorities. The Centre is financedby the Ministry of the Environment and the scientific work programme is followed by aninternational scientific advisory board.The overall scope of this project is to provide a science based input to the coming REACH reviewwith regard to endocrine disruptors. In accordance with the terms of reference the scientificevaluation includes a review of the paper on low dose effects and non-monotonic dose responses ofhormones and endocrine disrupting chemicals by Vandenberg et al. (2012) with regard to the levelof evidence for non-monotonic dose responses (NMDR).
2. Background and aimEndocrine disrupting substances are case-by-case covered by the authorisation scheme in REACH.If a substance is identified as an endocrine disruptor in accordance with Article 57 (f) anauthorisation can only be given if adequate control of the risk can be demonstrated. However,before June 2013 the European Commission is obliged to review REACH with regard to endocrinedisrupting substances. It is to be evaluated whether the application area for article 60.3 should beexpanded to include endocrine disrupting substances in general, which means that authorisation canonly be given if the socio-economic benefits “overrule” the risk and if there are no usefulsubstitutes.Only substances of particular concern are authorised via the socio-economic route, i.e. CMRs(Carcinogens, Mutagens, Reproductive toxicants) and substances of equivalent concern for which athreshold cannot be determined, PBTs (Persistent, Bioaccumulating, Toxic substances) or vPvBs(very Persistent and very Bioaccumulating substances) and substances of equivalent concern due toPBT or vPvB properties.It is therefore relevant to examine if a threshold for endocrine disrupters (EDs) in general can beestablished with reasonable certainty based on current scientific knowledge on the mechanisms andmodes of action for EDs - also taking into account current scientific knowledge on the possible so-called “low dose effects” and non-monotonic dose responses for EDs. If an assumption of thresholdseems plausible, it is furthermore relevant to examine whether the currently available and usedregulatory test methods are sufficiently sensitive for deriving a robust NOAEL (No Observed
4Adverse Effect Level) or BMD (Bench Mark Dose) in relation to endocrine relevant adverse effectsendpoints.The so-called “low dose effects” and non-monotonic dose-response for EDs have been underdiscussion for several years. There are different evaluations among researchers in the field on thequality and the extent of evidence in relation to these topics. The discussion as well as the numberof research papers has increased during recent years and in the beginning of 2012 a large review onlow dose effects and non-monotonic dose responses with focus on human health effects waspublished in the journal ”Endocrine Reviews” (Vandenberg et al. 2012). This review was animportant background paper for discussions at an EFSA colloquium on low-dose effects, June 12-15 2012, and at a joint EU/US workshop on low-dose effects and non-monotonic dose-response forendocrine active chemicals, September 11-13 2012, in Berlin. Some of the authors of this reportparticipated in both workshops and also contributed to the planning of the joint EU/US workshop.It is further relevant to evaluate whether EDs in general give rise to particular concern as the PBTs(persistent, bioaccumulating and toxic substances) or vPvBs (very persistent and verybioaccumulating substances) because this may or may not further support the management of EDsin accordance with REACH art. 60.3The aim of this report is, from a scientific point of view, to discuss the topics expected to berelevant for the REACH review on EDs, i.e.:- Thresholds or non-threshold assumption for ED effects- Considerations concerning non-monotonic dose-response (NMDR)- Uncertainties of the currently regulatory test methods with regard to determination ofpossible thresholds for EDs- Whether there is particular concern for EDsThe REACH review on endocrine disruptors considers both human health and the environment. Thefocus in this report is, however, restricted to human health as both the review from Vandenberg etal. (2012) and the workshops mentioned above focused on human health. The report does notspecifically discuss the so-called ‘low dose effects’ of EDs. However, this topic is indirectlycovered in the sections on thresholds, NMDR and uncertainties.
3. Threshold for EDsOne of the key concepts in toxicology and risk assessment is the dose-threshold, which implies thatchemicals can only cause (non-cancer) effects above a certain dose level (Slob 1999).Historically, dose-response assessments have been conducted differently for cancer and non-cancereffects. For carcinogenic effects, it has earlier on, generally been assumed that there is no dose-threshold for effect, and dose-response assessments have focused on quantifying risk at low doses.However, during the last decade considerations of mode of action have highlighted that there maybe a need to differentiate the approaches for genotoxic and non-genotoxic carcinogens. For non-
5cancer effects, a dose-threshold has been assumed and no observed adverse effect levels (NOAEL)or benchmark doses (BMD) have been used as a point of departure for deriving levels below whicheffects are not expected to occur or are extremely unlikely in an exposed population (Abt et al.2010). Evidence of mutagenicity is used to differentiate between genotoxic and non-genotoxiccarcinogens and for genotoxic carcinogens the assumption of non-threshold effects precludes theestablishment of a Derived No Effect Level (DNEL) (ECHA 2007).It is currently discussed whether an assumption of non-threshold may also be valid for chemicalswith endocrine mode of action. Also, the existence of non-monotonic dose response for EDs iscurrently discussed and this issue is dealt with in section 4. In this section, focus is on threshold ornon-threshold at the low end of the dose-response curve irrespective of whether this is a monotonicor non-monotonic dose-response.3.1 What is a threshold?The threshold for effect may be defined in different ways, which may be relevant to the argumentsfor or against a threshold for EDCs. Slob (1999) provided three different definitions:1. Mathematical definition: the dose below which the response is zero and above which it isnon-zero.2. Biological definition: the dose below which the organism does not suffer from any(adverse) effect.3. Experimental definition: the dose below which no effects are observed.The presence or absence of a threshold using the mathematical definition can never beexperimentally proven or ruled out (Kortenkamp et al. 2012; Sheehan et al. 1999; Slob 1999). Allmethods for measuring effects have a limit of detection below which effects cannot be observed,which will obscure thresholds, if they exist (Kortenkamp et al. 2012). Also, to generate an exactdose-response curve would require an infinite number of doses and infinitely precise measures(Slob 1999). Additional complicating factors are related to normal biological variation and thelimited power that is available with the size of dose groups normally used in toxicity testing (seesection 5).As it is not possible to experimentally prove the existence or absence of a threshold, evaluations onwhether effects of EDs should be assumed to exhibit a threshold or not have to be based on acombination of biological plausibility and experimental observations.3.2 Biological thresholds for EDs?The reviewed literature provides arguments both for and against assuming a threshold for EDs. Thegeneral argument for assuming no biological threshold for EDCs is that because low doses ofendogenous hormones are present and fluctuating, small additions (or subtractions) to their actionswill have a significant impact (Zoeller et al. 2012). This “additivity-to-background” argument hasalso been made to defend a no-threshold-approach for genotoxic carcinogens (Slob 1999).
6A central tenet of endocrinology is that hormones exert their physiological actions throughreceptors (Zoeller et al. 2012). This has several implications. First, hormone action is saturable interms of both ligand-binding and effect. Moreover, the maximum effect of the hormone typicallyoccurs at ligand concentrations well below those that result in receptor saturation. Theseobservations impose several consequences for the expected shape of dose-response curves inducedby hormones and by chemicals that interfere with hormone actions. First, the curves are neverlinear, although they may contain linear portions. Instead, they tend to be sigmoidal in shape butmay depart from this basic form, as in the case of non-monotonic dose-responses (see section 4). Itis the nature of sigmoidal dose responses that an equivalent change in hormone level (or action) atboth the very low end and the high end of the curve will have a small effect, whereas at the mid partof the curve the effect is proportionally greater. Furthermore, because low doses of endogenoushormones are present and fluctuating, small additions (or subtractions) to their actions will have animpact (Zoeller et al. 2012, Kortenkamp et al. 2012, Vandenberg et al. 2012). If no homeostaticcontrol occurs, this implies that endocrine disrupting chemicals can exhibit activity in a threshold-independent fashion. On the contrary, if homeostatic control occurs like protein binding ofhormones or chemicals, buffering of hormone levels via feed-back mechanisms etc., a threshold ofEDCs could be expected. It is important to note, that the presence of thresholds can never beconfirmed or rejected by experimental data as indicated in the previous section.The arguments made in support of a biological threshold for EDs are mainly that, as stated forexample by Blair et al. (2001): “…a threshold could be expected if there is no endogenoushormone, if the endogenous hormone induces no adverse effect, or if there is effective homeostaticcontrol”. This may be of relevance for the function of hormones in adults, where there may be aneffective homeostatic control. However, during development hormones have a very importantorganizing role in relation to the sexual dimorphic development of the reproductive system and thebrain, the general development of the brain (e.g. thyroid hormones) and also for foetal programingof the endocrine system (Kortenkamp et al. 2012). Thus, during development, endogenoushormones are present and “wrong” levels of endogenous hormones may induce adverse effects. Inhumans, hormonal regulations and feedback interactions develop during foetal life and for thehypothalamus-pituitary axes this system is functional after 20 weeks of gestation (Siler-Khordr1998). The steroidogenesis of androgens and oestrogens, however, occurs earlier and organizes thesexual dimorphic development of the reproductive system during 7-10 weeks of gestation (Moore1983). This implies that during sensitive windows of prenatal development there is no effectivehomeostatic control, because the buffering of hormone levels via feed-back mechanisms is notdeveloped yet. In conclusion, the above mentioned arguments for a biological threshold are notrelevant during sexual development.Conolly and Lutz (2004) state that the first interaction of a toxic agent with its primary biologicaltarget molecule is likely to have no threshold but imply that the complexity of a biological systemmakes non-threshold dose-response curves unlikely for many “higher” endpoints, such asbehaviour, reproduction, organ weights and growth. In relation to effects of EDs, this would meanthat although there is not necessarily a threshold for the primary biological action, the integration ofchemical influences on several pathways of importance to development of a certain ”higher” type ofeffect may lead to threshold-like response patterns. This may be the case when e.g. opposing effects
7occur at different dose levels due to different specific mechanisms of action occurring, and theinfluence of one direction of effect overrides the opposing effect caused by another mechanism ofaction of the same compound. This point is related to the presence of non-monotonous dose-response curves discussed in section 4.3.3 Do toxicological data indicate threshold or no-threshold?Probably, because of its suggestive wording, the term NOAEL may be taken to imply an absence ofeffects, as expressed for example by Ashby et al. (2004): “If the statistical methods used areappropriate, the absence of significance should indicate the absence of a chemically induced effect”(as described in Scholze and Kortenkamp 2007). The term NOAEL is, however, not the same as athreshold, because a NOAEL as signalled by the O for “observed” is the dose level where no effectsare observed and thus depend on the sensitivity of the methods for assessing the effects (see section5).To examine the threshold assumption for endocrine active chemicals with non-genotoxic endpoints,Sheehan (2006) examined dose-response data from the literature and the hypothesis was that nothreshold exists when a substance acts through the same mechanism as endogenous oestradiol, i.e.has oestrogenic activity. The analysis was accomplished by fitting the dose-response data to amodified Michaelis-Menten equation, which has no threshold term. Thirty-one data sets fromstudies on 9 different substances were evaluated. The chemicals used included natural (oestradiol)and synthetic (e.g., diethylstilbestrol and conjugated oestrogens) hormones as well as severalsynthetic endocrine disruptors (e.g. dioxin, polychlorobiphenyls). Twenty-six of the data sets fittedthe modified Michaelis-Menten equation with high multiple correlation coefficients (r>0.90). Theendpoints included both physiological (e.g. plasma prolactin levels and cell proliferation), andadverse responses (e.g. presence of vaginal threads and adenomas). Sheehan (2006) state that it isnot surprising to observe a good fit to the modified Michaelis-Menten equation without a thresholdterm for many of the examined dose-response data, since endocrine disruptors are capable of actingthrough receptor binding initiating a rate-limiting step that does not exhibit a threshold.In the US NTP low dose peer review report (Melnick et al. 2002) it was evaluated that forfinasteride, which acts as a 5α–reductase inhibitor, the dose-response curve for reduction in maleanogenital distance (linear) was different from that for increased hypospadias (threshold-appearing).Also, exposure of pregnant rats to vinclozolin at six doses ranging from 3.125 to 100 mg/kg/dayresulted in reduced anogenital distance and increased incidences of areolas and nipple retention inmale offspring (Melnick et al.2002). For these effects, the dose–response curves appeared linear tothe lowest dose tested. Reproductive tract malformations and reduced ejaculated sperm numberswere observed only at the two highest doses. These observations indicate that the shape of the dose–response curves may be low-dose linear for the effects on anogenital distance and nipple retention.In relation to hypospadias, the threshold-appearing response might indicate a threshold, oralternatively it may reflect the limited sensitivity for detecting rare quantal effects (see section 5).Thus, based on these data it is evaluated as uncertain whether there is a threshold or not forhypospadias.
8
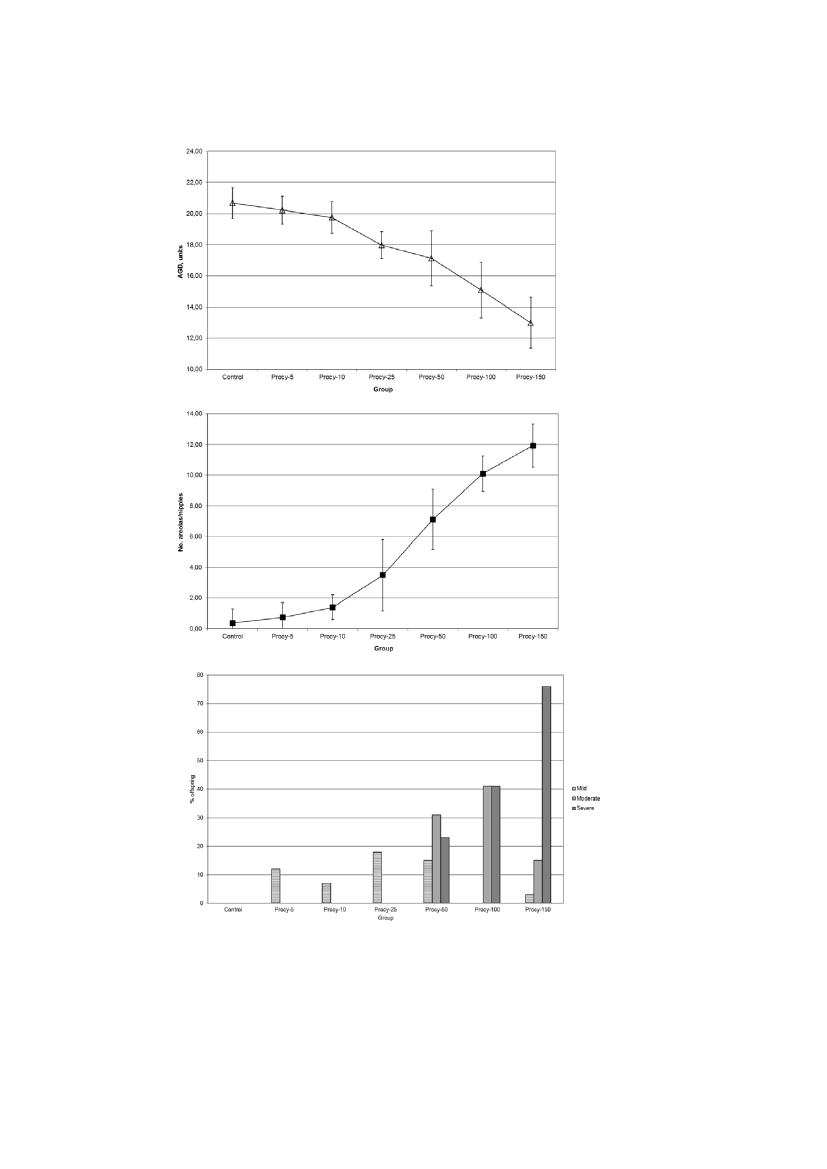
Figure 1. Dose-related decrease in anogenital distance day 1 (top), increase in nipple retention day 13 (middle) anddysgenesis of external sex organs day 16 in male offspring exposed perinatally to procymidone. Results shown foranogenital distance and nipple retention are mean + SD. For genital dysgenesis, the % offspring affected is shown.Based on Hass et al. 2007 and Metzdorff et al. 2007. the dose response curves appeared non-thresholded for AGD andnipple retention, but threshold-like for genital dysgenesis
9
Similar results as described above have been found in studies of the effects of the AR-antagonistsflutamide, vinclozolin and procymidone on male sexual development (Hass et al. 2007, Metzdorffet al. 2007). At the doses studied, the dose-response curves appeared non-thresholded for AGD andnipple retention, but threshold-like for genital dysgenesis (hypospadias), see figure 1 where theresults for procymidone are shown. These dose-response data were all fitted to nonlinear sigmoidalmodels. The arguments for not using a threshold model parameter were to keep the modellingsimple and robust but also more importantly, that none of the data analysed justified inclusion of athreshold parameter (Martin Scholze, pers. com). Based on this, it is not possible to concludewhether the dose-response may have a threshold or not, i.e. it is simply unknown.The problem of methodological limitations has made it difficult to reach conclusions aboutadditivity to the background concerning morphological effects because it has not been possible todesign experiments that have sufficient sensitivity to determine whether very small doses of acompound have any effect (Boobis et al. 2009). Gene expression has been analyzed in foetal rattestis exposed transplacentally to three different compounds with estrogenic activity - ethinyloestradiol, genistein, and bisphenol A. Doses for each compound spanned five or six orders ofmagnitude, starting from a dosage known to have pharmacological activity, down to very low doselevels. All three compounds had effects on gene expression at the higher dose levels, and there werestill some effects on gene expression at doses lower than those that had morphological effects;however, at the lowest dose levels of these compounds there were no significant changes from thecontrol in relation to gene expression (Naciff et al., 2005b as described in Boobis et al. 2009).According to Boobis et al. (2009), this result strongly suggests a threshold for activity of oestrogenson gene expression during development. However, we find that similarly as for morphologicaleffects such as anogenital distance it is not possible to determine whether the effect on geneexpression effect actually has a threshold or the results just showed a threshold-like dose-response,because the variability in the measurement overwhelmed the ability to detect very small changes ina reasonable number of animals. Therefore, based on these data, we conclude that effects on geneexpression appear to be more sensitive endpoints than morphological effects, but there areuncertainties with regard to threshold for both types of effects.3.4 ConclusionsThe presence of thresholds can never be confirmed or rejected by experimental data, because allmethods for measuring effects have a limit of detection below which effects cannot be observed.Thus evaluations on whether effects of EDs should be assumed to exhibit a threshold or not have tobe based on a combination of biological plausibility and experimental observations.A general argument for assuming no biological threshold for EDCs is that because low doses ofendogenous hormones are present and fluctuating, small additions (or subtractions) to their actionswill have a significant impact. The validity of assuming no biological threshold for EDs issupported by the very important organizing role of hormones during development at a time pointwhere the homeostatic control is not effective or not developed yet. Also, experimental data indicatenon-thresholded dose-response for some endpoints for adverse effects on sexual differentiation suchas anogenital distance and nipple retention at the dose levels studied so far. It is therefore concluded
10based on a combination of biological plausibility and experimental observations that an assumptionof no threshold appears more valid for the effects of EDs during development than an assumption ofa threshold.Regardless of ED mode of action, it is uncertain whether or not there is a threshold for EDs. ForEDs, where the MoA (Mode of Action) directly involve the receptor, the interaction with thereceptor is likely to have no threshold. For EDs affecting the hormone levels, the response patternmay appear threshold-like, because multiple pathways converge before seeing the final responseand some of these pathways may have a threshold.Irrespective of threshold or non-threshold, the dose response curves of EDs seem generally to bebest described as sigmoid curves, i.e. the effect decreases asymptotically with dose towards zero butdoes not become zero, as supported by several types of experimental data. Such curves, however,have a “threshold-like” appearance, but a threshold cannot be inferred from the shape of the dose-response curves. However, a benchmark approach may be used for estimating a human exposurelevel with very low risk.
4. Non-monotonic dose-response (NMDR) for EDsIn the fields of toxicology and human health-risk assessment there is currently much debate aboutthe shape of the dose-response curve. By a monotonic dose-response, the observed effects may belinear or non-linear, but the slope does not change sign. In contrast, a dose-response curve is non-monotonic when the slope of the curve changes sign somewhere within the range of dosesexamined (Vandenberg et al. 2012). NMDRs are often U-shaped (with maximal responses of themeasured endpoint observed at low and high doses) or inverted U-shaped (with maximal responsesobserved at intermediate doses). Numerous toxicological studies show a NMDR curve with either adecrease in the response below control at low dose followed by an increase at high dose (U- or J-shaped) orvice versa(inverted U- or β-shape) (Conolly & Lutz, 2012).4.1 ED mechanisms for NMDRThere are several mechanisms that illustrate how hormones and EDs may cause NMDRs. Thesemechanisms include cytotoxicity, cell and tissue-specific receptors and cofactors, receptorselectivity, receptor down-regulation and desensitization, receptor competition, and endocrinenegative feedback loops (Vandenberg et al. 2012). In the following, these mechanisms are brieflydescribed based on Vandenberg et al. (2012) with main focus on those mechanisms where theNMDR can be related to functions of the endocrine system. For further details and specificreferences, see Vandenberg et al. 2012.CytotoxicityThe simplest mechanism for NMDR derives from the observation that hormones can be acutelytoxic at high doses yet alter biological endpoints at lower doses. As experimental results clearly
11indicate that the effects of for example oestradiol at high doses are due to toxicity via non-ER-mediated mechanisms we do not consider such NMDRs as evidence for endocrine related NMDR.Cell- and tissue-specific receptors and cofactorsSome NMDRs may be due to the combination of two or more monotonic responses that overlap,affecting a common endpoint in opposite ways via different pathways. For example, oestrogenshave been shown to induce cell proliferation and inhibit apoptosis in several cell populations, butinhibit proliferation and induce apoptosis in others, with the combined effect being an inverted U-shaped curve for cell number. In many cases, it is difficult to evaluate whether observed NMDR foran ED endpoint is due to two or more monotonic endocrine related responses as mechanistic data isscarce. In the absence of mechanistic data, it is proposed to assume that such NMDRs areconsidered as evidence for endocrine related NMDR until proven otherwise.Receptor selectivityNMDRs can occur because of differences in receptor affinity, and thus the selectivity of theresponse, at lowvs.high doses. Thus, the effects seen at high doses may be due to action via thebinding of multiple receptors in contrast to the effects of low doses, which may be caused by actionvia only a single receptor or receptor family. If NMDR is seen due to such action this is evaluatedas clearly related to the function of the endocrine system.Receptor down-regulation and desensitizationWhen hormones bind to nuclear receptors, the outcome is a change in the transcription of targetgenes. After this, the reaction must cease;i.e.the bound receptor must be inactivated in some way.Nuclear hormone receptors can be degraded and as hormone levels rise, the number of receptorsbeing inactivated and degraded also rises, and the number of new receptors being produced may notmaintain the pace of the degradation.There can also be receptor desensitization, where a decrease in response to a hormone is due tobiochemical inactivation of a receptor. Desensitization typically occurs when repeated orcontinuous exposure to the ligand occurs. Receptor desensitization has been observed for a range ofhormones including glucagon, FSH, human chorionic gonadotropin, and prostaglandins.Receptor down-regulation and desensitization may occur in the same cells for the same receptor,and therefore, both can play a role in the production of NMDRs. In such cases, NMDR is related tothe function of the endocrine system.Receptor competitionMathematical modelling studies suggest that endogenous hormones and EDs establishes a naturalenvironment to foster NMDRs. Using mathematical models, Kohn and Melnick (as described inVandenberg et al. 2012) proposed that when ED exposures occur in the presence of endogenoushormone and unoccupied hormone receptors, some unoccupied receptors become bound with theED, leading to an increase in biological response. At low concentrations, both the endogenoushormone and the ED bind to receptors and activate this response, but at high doses, the ED mayoutcompete the natural ligand. The model predicts that inverted U-shaped curves may occur and
12would be abolished only if the concentration of natural hormone were raised such that all receptorswere bound.Endocrine negative feedback loopsIn several cases, the control of hormone synthesis is regulated by a series of positive- and negativefeedback loops. Studies indicate that these negative feedback loops could produce NMDRs whenthe duration of hormone administration is changed. For example, short exposures of oestrogeninduce proliferation in the uterus and pituitary, but longer hormone regimens inhibit cellproliferation. Thus, the exposure to a single hormone concentration – or an ED - may stimulate anendpoint until negative feedback loops are induced and the stimulation ends. As endocrine feed-back loops are not developed before the late part of foetal life (e.g. around week 20), NMDRs dueto this function of the endocrine system is not to be expected during foetal life.4.2 Human evidenceThe existence of NMDRs for endocrine active drugs has been recognized and used in humanclinical practice for many years (Vandenberg et al. 2012, Juul et al. pers. com). A different specificterm, i.e. flare, may be used. Flare is often reported in the therapy of hormone-dependent cancerssuch as breast and prostate cancer. Tamoxifen flare was described and named as a transientworsening of the symptoms of advanced breast cancer seen shortly after the initiation of therapy insome patients. If the therapy could be continued, the patients showing tamoxifen flare demonstrateda very high likelihood of subsequent response to tamoxifen, including arrest of tumour growth andprogression of symptoms for some time. The recognition of this dual dose-response range fortamoxifen led to the definition of the term selective oestrogen response modulator or SERM,activity. These observations defined three separate dose-response ranges for tamoxifen in humanclinical use. The lowest dose-response range, the range of flare, stimulated breast cancer growth andsymptoms in some patients with hormone-dependent cancer. The next higher dose-response range isthe therapeutic range where tamoxifen inhibits oestrogen-dependent tumour growth and the highestdose range causes acute toxicity by the SERM (Vandenberg et al. 2012).4.3 NMDRin vitroU-shaped or inverted U-shaped dose-response curves are often observed inin vitrostudies, which isusually due to various mechanisms of actions involved for the same chemical. The typical situationis a low concentration effect due to the primary mechanism tested and cytotoxicity (i.e. cell death)at higher concentrations. However, other cases exist in which two or more mechanisms of actionthat do not include cytotoxicity are into play.One example is seen for the antagonistic effect of hydroxyflutamide on the androgen receptor.Hydroxyflutamide is the hydroxyl-metabolite of flutamide, which is a drug used for treatment ofprostate cancer. A non-monotonic dose-response curve for androgen-receptor-mediated genetranscription by hydroxyflutamide was seen in HepG2 human hepatoma cells. This effect is ageneral phenomenon happening in several androgen receptor reporter gene assays. Low
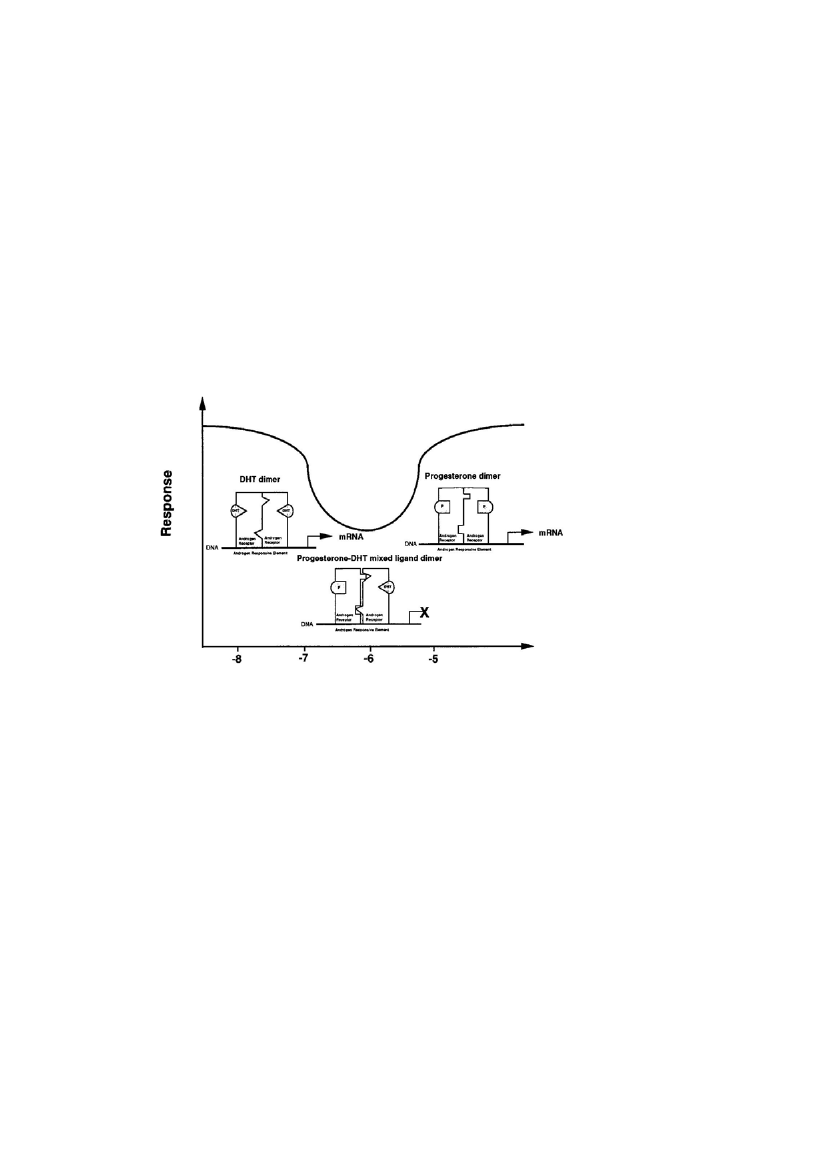
13hydroxyflutamide concentrations partially antagonized the effect of dihydroxytestosterone, whileagonistic activity was observed with a further increase in hydroxyflutamide concentration. Thebiphasic dose-response curve was explained by the hypothesis that only the receptor dimer thatcarry two DHT or two hydroxyflutamide ligands, but not mixed-ligand dimers, are transcriptionallyactive (Maness et al., 1998). Such a dose-response relationship for antagonism of the androgenreceptor is found for vinclozolin and progesterone as well. The mechanism of progesterone isbelieved to be comparable to that of hydroxyflutamide and this is illustrated in Figure 2.Another example is the antagonistic action of two adenosine receptor subtypes that regulateadenylate cyclase in opposite directions, given appropriate differences in ligand affinity and inefficacy of signal transduction, resulting in a clearly biphasic dose-response curve (Ebersolt et al.,1983). Many other examples fromin vitroandin vivostudies can be presented and explained asevidence for the existence of NMDRs.
Figure 2: Mixed-ligand hypothesis: Formation of ligand dimers and the resultant response for low, medium, and highconcentrations of progesterone in combination with an inducing concentration of dihydrotestosterone. With lowconcentrations of progesterone DHT–DHT dimers are more likely to form and induce a response. As the concentrationof progesterone increases, mixed progesterone–DHT ligand dimers form, which block androgen receptor activity. Athigh progesterone concentrations progesterone–progesterone dimers are more likely to form and induce a response.From Maness et al.(1998).
4.3.1 Critical review of in vitro studies included in Vandenberg et al. 2012In the review by Vandenberg et al., (2012) a comprehensive table summarizing results ofin vitrostudies giving rise to NMDR is presented. The definition of a NMDR used in this review is basedupon the mathematical definition of non-monotonicity: that the slope of the dose-response curvechanges sign from positive to negative orvice versaat some point along the range of dosesexamined. There are several adequate studies in the table that add confirmation for the hypothesisthat EDs are capable of eliciting NMDRin vitroincluding Jeng et al., (2009), Boettcher et al.,(2011), and Almstrup et al., (2002) (ref. 744, 719 and 730 in the Vandenberg review).However, there are several important points worth emphasizing regarding the criteria used forincluding many of thein vitrostudies to a list of studies showing NMDR. The broad definition usedto define NMDRs does not seem to distinguish between the mechanisms that underlie the curve i.e.
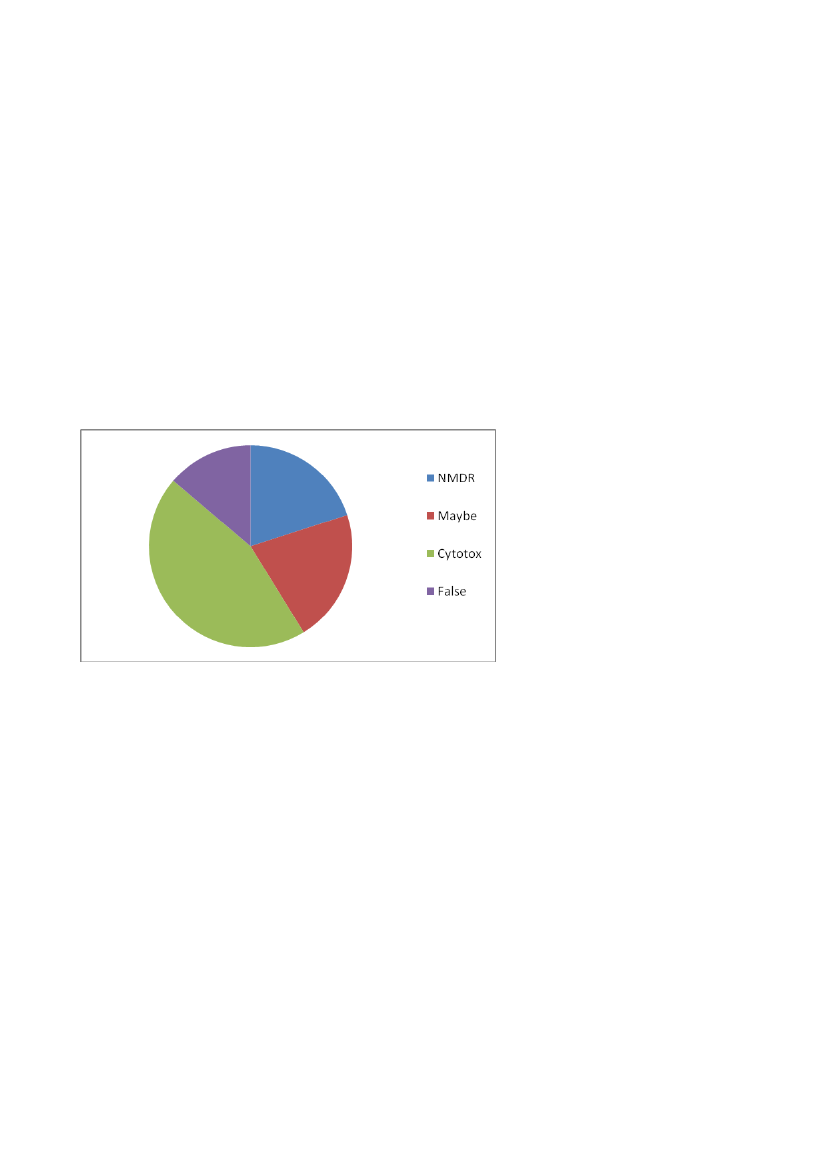
14the definition also allows for inclusion of studies with inverted U-shaped dose-response curves,which are the result of cytotoxicity at high concentrations. Thus, there are some studies, which haveshown cytotoxicity at the highest concentrations and even discuss its importance to the shape of thebiphasic curve (e.g. Asp et al., (2010) (ref. 754 in the Vandenberg review), and Alm et al., (2008)).In relation toin vitroinvestigations, inverted U-shaped curves caused by general toxicity towardsthe cells should not be regarded as “true” NMDRs, as this is merely a reflection of the concentrationlevel of the compound. If the definition is applied as suggested by Vandenberg et al., mostcompounds will give rise to NMDRs. Thus the definition used in the review by Vandenberg et al. isin our view too broad to be applied onin vitrostudies.Another important point to mention is the testing of hypotheses and the associated statistics. Manyof the studies used ANOVA followed by Dunnett’s post hoc test to test for significant differencesbetween the control and test concentrations. Yet, to demonstrate a NMDR according to thedefinition, a different kind of statistics e.g. testing for significant positive/negative slopes on eitherside of the curve peak to determine whether there is a real shift in the slope of the curve, will haveto be applied. In other words, to significantly demonstrate a NMDR, a completely differentapproach in hypothesis testing and statistics will have to be used. Somjen et al., (1998) (ref. 721 inthe Vandenberg review) describe the creatine kinase specific activity in vascular smooth musclecells as a result of increased concentrations of ethinyl oestradiol. The curve first shows a slightdecrease followed by an increase in activity and then a minor drop that might be due to cytotoxicity.The first point may be a coincidence and due to simple variation, since no statistical significancewas found. Similarly, no evidence has been provided that there was a real decrease in the curve atthe last point, since the statistics said nothing about the difference between the 10 nM and 100 nMconcentrations.In contrast, Leung et al., (2008) (ref. 728 in the Vandenberg review), compared all concentrationsin the dose-response curve showing the IGF-1 expression as a result of growth hormone exposureusing a Student–Newman–Keuls test. However, there was no significant difference between thethree highest concentrations (i.e. 10, 100, and 1000 ng/mL). The change in slope was notsignificant, but only due to random variation. So even though the statistic that was used here isbetter suited for the detection of biphasic curves, it could not be proven that this was in fact aNMDR.Lastly, NMDR may be the result of several mechanisms coming into play. Chemical mixtures canconsist of substances that possess different modes of action, and can therefore interfere with theinvitroassay in many different ways. Thus, studies investigating mixtures are not very suitable forevaluating the existence of NMDRin vitro.Again, a too broad definition was in our opinion usedwhen including mixture studies that showed biphasic dose-response curvesin vitro.Mixture studieslisted in the Vandenberg-review as showing NMDRsin vitroinclude Campagna et al., (2007) andOhlsson et al., (2010) (ref. 757 and 750 in the Vandenberg review).Figure 3 is a diagram showing the number of examples from the Vandenberg review describingNMDRin vitro(n=80 totally) allocated into four groups based on an evaluation according to ourdefinition, i.e. NMDR where cytotoxicity cannot explain the change in curve slope (blue), NMDR
15where cytotoxicity is or might be the cause of the change in the slope of the curve (green), NMDRthat for some of the above reasons may or may not be evidence for NMDR (red), and no evidencefor NMDR of EDs (purple). Almost half of the examples (45%) could not according to ourdefinition be regarded as showing a true non-monotonic dose-response, as the NMDR wasevaluated as due to cytotoxicity. Furthermore, some examples were evaluated as “false NMDR”,because of e.g. testing of mixtures or limitations in the study design. The remaining examples wereevaluated to either show evidence for NMDR (16%) or a dose-response that may or may not be dueto NMDR of EDs (17%). More details can be found in Appendix 1.In conclusion, the Vandenberg et al. review gives several good examples showing the existence ofNMDRsin vitro.Thus, there are well-conducted studies showing biphasic curve patterns, which aresupported by possible explanatory models. However, our critical evaluation based on the use of aless broad definition of NMDR leads to fewer cases than those included in the Vandenberg et al.review.
Figure 3: Pie chart showing the studies from the Vandenberg-review described as showing NMDRin vitroallocatedinto four groups. See text for further explanation.
4.4 NMDRin vivo4.4.1 Critical review of in vivo studies included in Vandenberg et al. 2012The papers selected for a critical evaluation included 34 from table 7 in the Vandenberg review.The papers selected were the majority of the studies related to human toxicity, with main focus onstudies where the effects were regarded as sufficiently severe in relation to the definition of adverseeffects.The evaluations were based on a weight of evidence assessment and considered many aspects incl.the number of animals per group, the number of dose levels, the statistical significance of the effectsand the plausibility for NMDR based on mode of action consideration in the paper. The evaluationdid not include weight of evidence across papers for a specific chemical. Based on this, theevidence in the papers was allocated into one of 3 groups according to the level of evidence, i.e.:
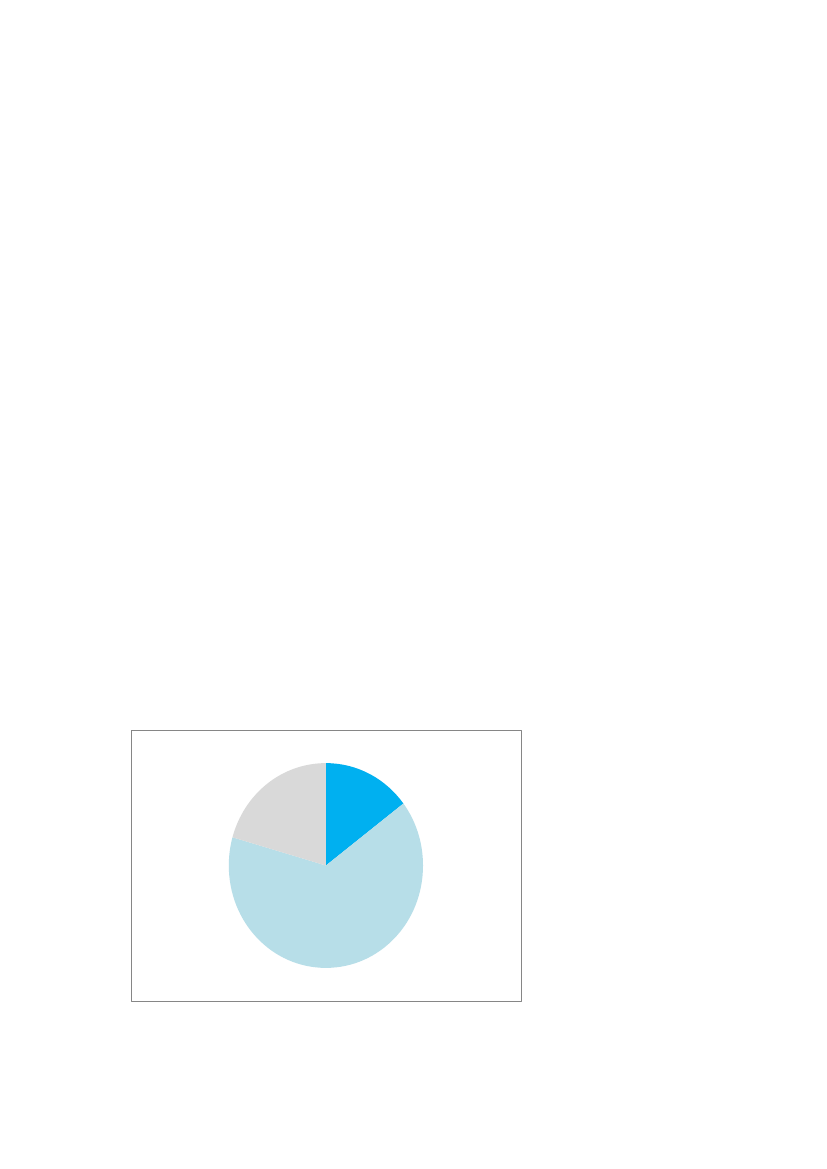
16Group1: Clear evidence for NMDR- the effects found can be regarded as adverse effects- a sufficient number of animals per group. This was not a fixed number as this depend on thepower for detection of the specific effects- statistically significant effect(s) at the peak (in case of inverted U-shaped dose-response) andalso a high plausibility of a significant difference between the effect at the peak and theeffect at higher dose(s)- plausible endocrine MoA(s) behind the observed NMDRGroup 2: Some evidence for NMDRMainly similar evidence as for group 1, but where there were limitations for some parts of theevidence needed for group 1.Group 3: Poor or no evidence for NMDR- insufficient number of animals leading to a the high probability for false-positive and false-negative findings- lack of statistical significance- the apparent NMDR was evaluated as due to general toxicity and thus not related to anendocrine MoA- use of an animal model deprived of the natural hormone and where the NMDR wasevaluated as due to normalization of the function followed by toxicity due to too highhormone level.Figure 4 is a diagram showing the number of examples evaluated (n=34 totally) grouped into thethree groups according to the level of evidence for NMDR based on our definition. The majority ofthe examples were evaluated to give some evidence for NMDR (n=22) and 5 studies showed clearevidence. Poor or no evidence for NMDR was concluded for 7 of the studies. More details can befound in Appendix 2.1. Clearevidence15%3. Pooror noevidence20%2. Someevidence65%
Figure 4: Pie chart showing 34 examples from the Vandenberg-review described as showing NMDRin vivogroupedinto three groups. See text for further explanation.
17
Our evaluation shows that there was clear experimental evidence for endocrine induced NMDR in alimited number of studies (5 studies), but also some evidence for NMDR in the majority of thestudies, i.e. 22 studies. For the latter studies more experimental data are needed to evaluate whetherthe observed NMDRs were actually real findings related to endocrine mode of action. However, wealso found poor or no evidence for some studies. This may reflect that a very broad definition wasused to define NMDR in the Vandenberg paper. For example, it seems that studies with inverted U-shaped dose-response curves, which are most likely the result of general toxicity at highconcentrations were are also included. Also, a few studies without any statistical significance wereincluded. Thus the broad definition apparently used by Vandenberg et al. was too broad in our viewto be correctly applied onin vivostudies. Nevertheless, our evaluation indicates that for themajority of the studies evaluated by Vandenberg et al. there was clear or some evidence for NMDR.In the following sections some of the studies in the Vandenberg-review as well some publishedresults from our own studies of endocrine disrupters that are not included in the Vandenberg paperare described and evaluated in relation to NMDR.4.4.1.1. NMDR and reproductive organ weightsFor reproductive organ weights, several cases of NMDR have been described. These curves couldeither be associated with differences in androgen action as described previously or could reflecthow effects in the target organs are interrelated and cause changes in organ weights in one directionat low doses, and another effect at higher doses. This happens when another action of the compoundappears and affects the organ in the opposite direction. For example, testis weight can be affectedby chemically induced changes such as fluid accumulation or impaired proliferation/differentiation,and these changes will likely have opposing effects on testis weight. If these changes appear atdifferent doses, it may be speculated that this could result in NMDR curves for testis weight.-One example of NMDR has been found for the effects of procymidone on testis weight intwo different studies. The first study showed no change in testis weight at 5 mg/kg bw/day,a statistically significant increase at 10 mg/kg bw/day, no change at 25, 50 and 100 mg/kgbw/day, and a statistically significant decrease at 150 mg/kg bw/day (Metzdorff et al.,2007). In another study from the same laboratory, a statistically significant increase in testisweights was observed in animals exposed to the lowest dose of 12.5 mg/kg bw/day ofprocymidone but not at 50 mg/kg bw/day (Jacobsen et al., 2012). As body weight was usedas a covariate in these studies, these changes were not caused by differences in body weight,but could rather reflect NMDR due to endocrine disrupting effects.
Prostate weight has also been shown to be affected by estrogenic compounds in a non-monotonousmanner.-Exposure of neonatal male rats to oestradiol benzoate resulted in increased prostate weightsat low doses (0.15 ug/kg bw) and decreased prostate weight at high doses (1500 and 15000ug/kg bw) when examined at PND 35 (Putz et al. 2001). In adulthood, a comparable patternof effects was seen, though only the weight reductions at high doses were statistically
18significant. The Putz et al. (2001) study was included in the Vandenberg paper (ref. 780),and assessed by us as belonging to group 2.In mice exposed to diethylstilbestrol (DES) during gestation, low doses (0.02, 0.2 or 2 ug/kgbw) resulted in increased prostate weights and high dose exposure (200 ug/kg bw) resultedin reduced prostate weights in adulthood (Vom Saal et al. 1997). Likewise, low levels of17β- oestradiol increased prostate weights in adult mice exposed in utero, whereas prostateweights were unchanged at higher doses. Prostatic androgen receptor expression wasincreased at low levels of 17β-estradiol compared to controls. The Vom Saal et al. (1997)study was included in the Vandenberg paper (ref. 689), and assessed by us as belonging togroup 1.In androgen-responsive reporter mice exposed to hexachlorbenzene during gestation,lactation and prepuberty, an increase in prostate weight and androgenic activity was seen atlow doses, but not at high doses (Ralph et al., 2003). With continued exposure to 8 weeks, adecreased androgenic activity was seen at high doses. Low doses also increased epididymisand testis weights at 4 weeks and induced early puberty, while high doses showed no changeof epididymis or testis weights. (Ralph et al., 2003). The Ralph et al. (2003) study was onlyincluded in the Vandenberg paper (ref. 755) in table 6 (Examples of NMDRCs in cellculture experiments) and therefore the in vivo results from this study were not assessed byus in relation to our grouping of the in vivo examples.
-
-
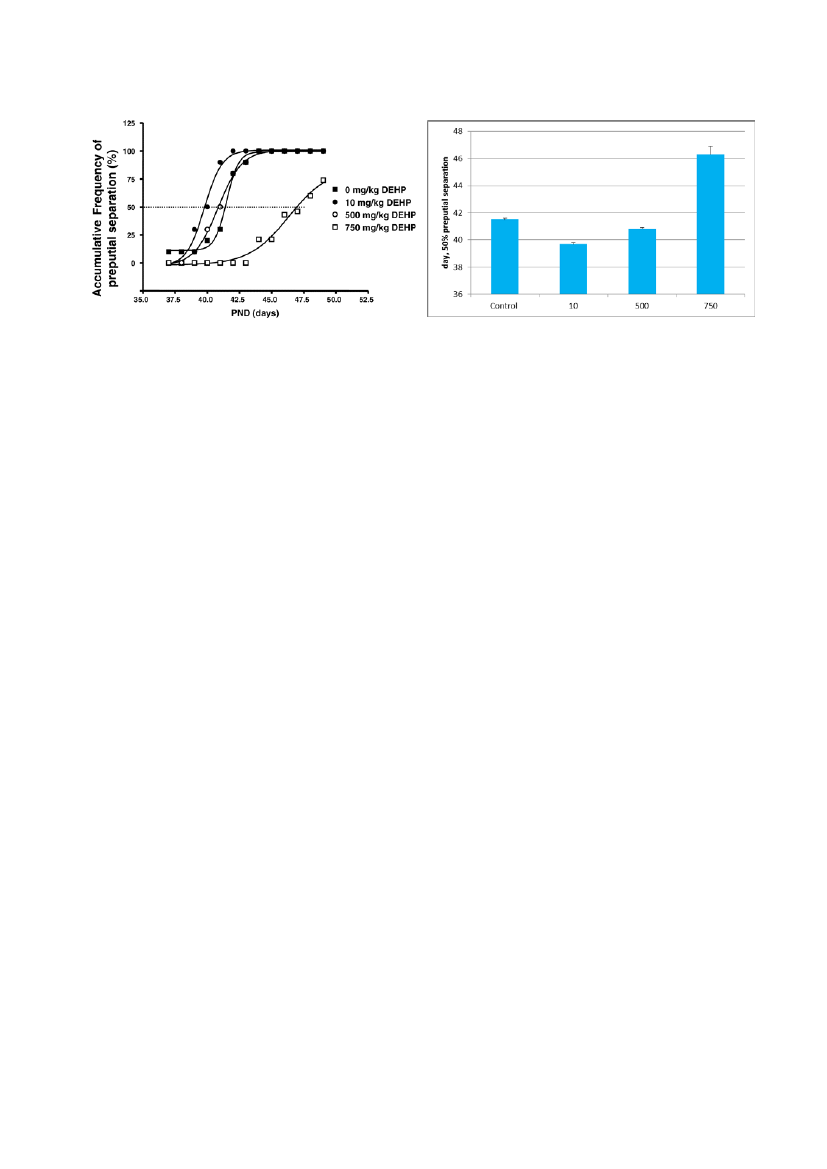
Early onset of puberty due to increased gonadotropin levels or altered sensitivity to androgens(e.g. increased androgen receptor expression) could be the cause of increased reproductiveorgan weights at low doses. At high doses, androgen receptor levels are down regulated and it issuggested that these opposing high- and low-doses effects are due to different modes of actionappearing at different dose levels. In contrast, the study on hexachlorbenzene (Ralph et al.,2003) indicates that there may also be cases when a non-monotonous response is caused by thesame primary effect/mode of action (androgen receptor interaction), and that the opposingresponses are due to the non-monotonicity of dose-response curves for partial agonists.4.4.1.2. NMDR and timing of puberty and nipple retention in male offspringExamples of NMDR have also been described for DEHP for two different endpoints, i.e.:preputial separation and nipple retention (Ge et al. 2007, Christiansen et al. 2010).The Ge et al. (2007) study was included in the Vandenberg paper (ref. 789), and assesses by usas belonging to group 1. Male Long-Evans rat pups were chronically subjected to low or highdoses of DEHP, with the androgen-driven process of preputial separation used as an index ofpubertal timing. The results are averages from 2 experiments. Rats were treated with 0, 10, 500,or 750 mg/kg body weight DEHP for 28 days starting at day 21 postpartum. The average age atwhich the animals completed preputial separation was recorded in each group. The age ofpreputial separation was 41.5 � 0.1 days postpartum in controls (vehicle). The 10 mg/kg DEHPdose advanced pubertal onset significantly to 39.7 � 0.1 days postpartum, whereas the 750mg/kg DEHP dose delayed pubertal onset to 46.3 � 0.1 days postpartum (see fig 5).
19
Figure 5. Left (from Ge et al. 2007): Biphasic effect of di(2-ethylhexyl)phthalate (DEHP) exposures on pubertyonset assessed by preputial separation. Prepubertal rats were gavaged with DEHP (0, 10, 500, and 750 mg/kg/d).The time course of the accumulative frequency of rats with preputial separation was fitted by sigmoid nonlinearregression. Average age was calculated as the intercept at 50% accumulative frequency, shown as the dotted line.Right: The same results shown as mean values + SEM according to the day of preputial separation.
Moreover, a similar picture was seen for body weight, seminal vesicle weight and serumtestosterone. The 10 mg/kg DEHP dose significantly increased serum testosterone (T) levels(3.13 � 0.37 ng/mL) and seminal vesicle weights (0.33 � 0.02 g) compared with control serum T(1.98 � 0.20 ng/mL) and seminal vesicle weight (0.26 � 0.02 g), while the 750 mg/kg dosedecreased serum T (1.18 � 0.18 ng/mL) as well as testes and body weights. The statistics arewell performed however the results are averages from 2 experiments. Thus, this paperdemonstrated NMDR as low-dose exposure to DEHP (10 mg/kg) induced increased serum Tlevels, precocious 2-day advancement in the timing of preputial separation, and increases inseminal vesicle weight in male rats, whereas higher doses of DEHP (750 mg/kg/d) had theopposite effect of lowering T levels and delaying puberty. However, it is important to keep inmind that the NMDR might be a secondary to the effect on bodyweight which follows the samepattern.In the studies reported in Christiansen et al. 2010, the effects of perinatal DEHP exposure wasstudied in time-mated Wistar rats gavaged from gestation day 7 to postnatal day 16 with 0, 10,30, 100, 300, 600 and 900 mg/kg bw/day (study 1) and 0, 3, 10, 30 and 100 mg/kg/day (study2), respectively. The results showed that DEHP at a relatively low dose of 10 mg/kg causedadverse anti-androgenic effects on male rat sexual development. At this dose level, maleanogenital distance was decreased, the incidence of nipple retention was increased, weights oflevator ani/bulbocavernosus muscle (LABC) were reduced and mild external genitaliadysgenesis was observed.
20
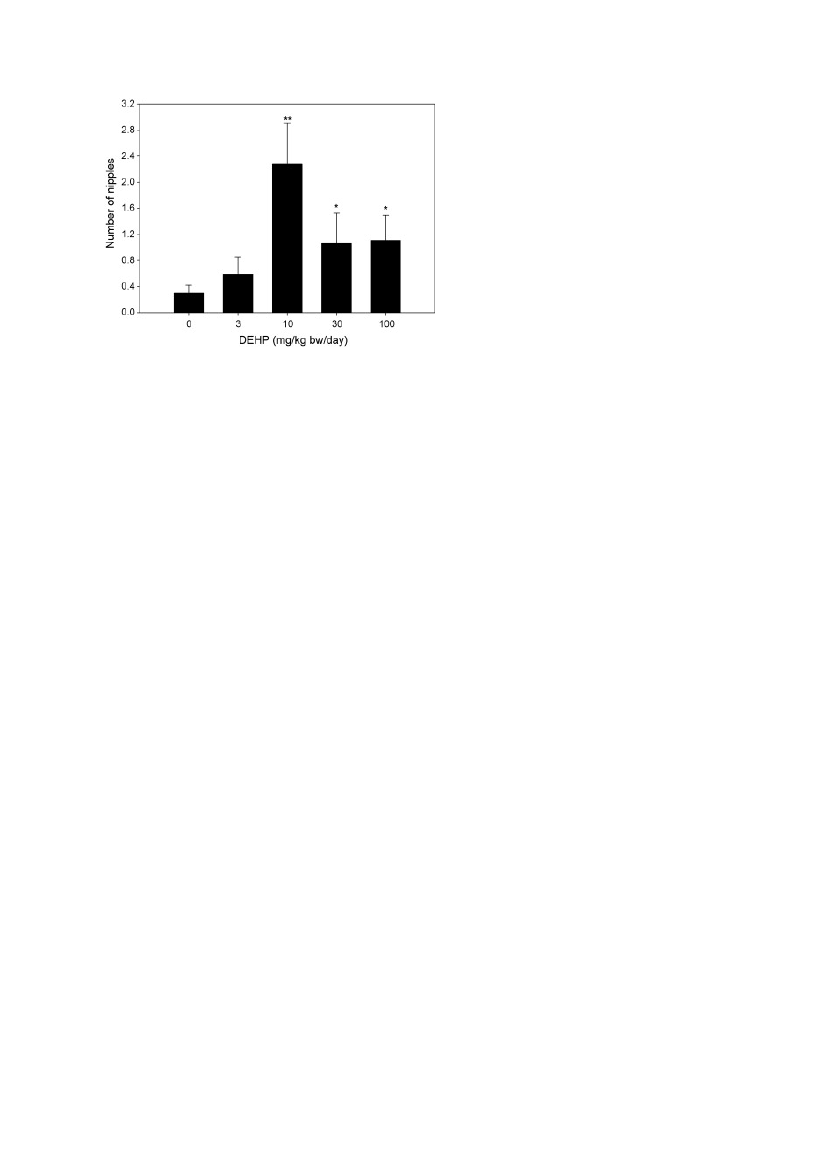
Figure. 6. Mean number of nipples in male rat offspring of dams exposed to corn oil (control), 3, 10, 30 or 100 mg/kg-dDEHP from GD 7 to PND 16. Results are based on analysis of litter means and are presented as mean + SEM. Datarepresents the combined analysis of study 1 and study 2. *Indicates p ≤ 0.05, **indicates p < 0.01.The exposure toDEHP statistically significantly increased nipple retention in male rat offspring at 10, 30, and 100 mg/kg-d (figureshown here) but also at 300, 600 and 900 mg/kg-d (not shown).
In study 1, perinatal DEHP exposure induced nipple retention in male offspring at all dose levels,i.e. from 10 mg/kg. However, the dose–response relationship seemed non-monotonic, as 10 mg/kginduced a more marked effect than 30 and 100 mg/kg. In study 2, there also seemed to be a highernumber of nipples at 10 mg/kg compared to controls, although the difference was not statisticallysignificant. A combined analysis of the data from both studies showed that at doses above 3 mg/kgincreased nipple retention was observed (figure 6). The combined dose–response curve alsoappeared as non-monotonic, as the dose of 10 mg/kg still seemed to induce a more marked effectthan 30 mg/kg (p = 0.053) and 100 mg/kg, though not statistically significant (Christiansen etal.2010).These DEHP results indicate NMDR in relation to nipple retention and a similar picture was seenfor the incidence of male offspring with mild external genital malformations, and reductions inweight of LABC, with more pronounced effects at 10 mg/kg than at higher doses. These endpointsare all recorded during the last part of the lactation period and the NMDR might therefore be due tospecial mechanisms or toxicokinetics during this period or it might be due to biological variationand incidental difference in response in animals from these groups. However, the existence of a bi-phasic dose–response pattern for DEHP cannot be excluded. Ge et al. (reported above) found a non-monotonous (biphasic) effect on sexual maturation when exposing male rats to DEHP in theprepubertal period (PND21-48) (Ge et al. 2007). Moreover, Andrade et al. (2006) also found aNMDR curve, i.e. a J-shaped curve where the aromatase activity was inhibited at low doses andincreased at high doses in DEHP exposed (GD6-PND21) male rats on PND1 (Andrade et al. 2006ref. 788 in Vandenberg et al). In the latter study, the decreased aromatase activity at 0.1 and 0.4mg/kg and increased at 15, 45, 135 and 405 mg/kg in male offspring may not be considered as anadverse effect, but more evidence for an ED mode of action. Taken together these three studiesindicate that DEHP induces NMDR for some ED endpoints.
214.5 ConclusionsThere are several mechanisms that illustrate how hormones and EDs may cause NMDRs due to thefunction of the endocrine system. These mechanisms include receptor selectivity, receptor down-regulation and desensitization, receptor competition, and endocrine negative feedback loops.NMDR for EDs exists and have been shown and used in human endocrinology as a basic principlebehind the pharmaceutical treatment of severe diseases. Also, NMDR has been shown for manydifferent ED-mediatedin vitroandin vivoeffects including binding to steroid hormone receptorsand adverse effects on reproductive organ weights (prostate and testis), nipple retention and sexualmaturation. In many of the cases the observed NMDR is likely to directly reflect the way theendocrine system works. In other cases, the NMDR may reflect that the substance has multiple EDmodes of action operating simultaneously, but with different dose-response curves. As detailedmechanistic knowledge is limited for most EDs it is often difficult to evaluate the MoA behindNMDR.
5. Uncertainties related to regulatory requirements and test methodsOne of the aims of the present report was also to give scientific input on the uncertainties of thecurrently used regulatory test methods, with regard to determination of possible thresholds for EDs.In regulatory practise NOAELs are generally used as part of risk assessment or as point of departurefor deriving acceptable human exposure levels. NOAELs are, however, not fixed values, but aresensitive to the specific features of the chosen experimental design, the choices of statisticalmethods and significance criteria. Thus, when there is no statistically significant difference inresponse between treated groups and controls, it can only be concluded that the magnitude of effectwas below the detection limit of the particular experimental arrangement used (Scholze andKortenkamp 2007).If the effects of EDs are to be identified within various kinds of regulations, including REACH, it isessential that the testing requirements include studies where the exposure covers windows ofincreased susceptibility and the relevant endpoints are assessed (Kortenkamp et al. 2012). Inaddition, it is important that the power for detecting a relevant threshold-like dose is sufficient forthe endpoints assessed.5.1 Current REACH information requirements, test methodsThe information requirements for substances for registration under REACH are differentiatedaccording to supply tonnage. Generally, testing requirements at a lower tonnage level apply to thehigher tonnage level, unless exemptions are clearly stated. The current information requirements inREACH is not designed for the identification of endocrine disrupters, but some relevant testmethods for detection of endocrine disrupters are mentioned in relation to testing for repeated dose
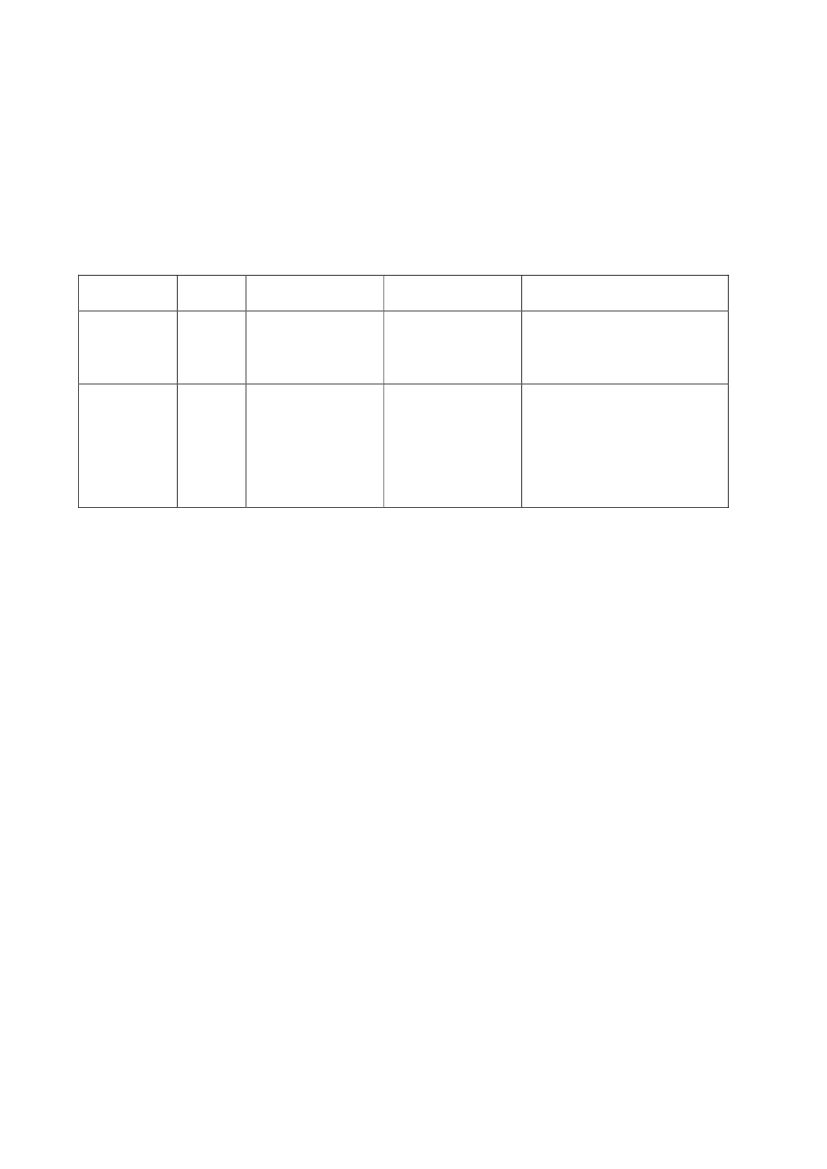
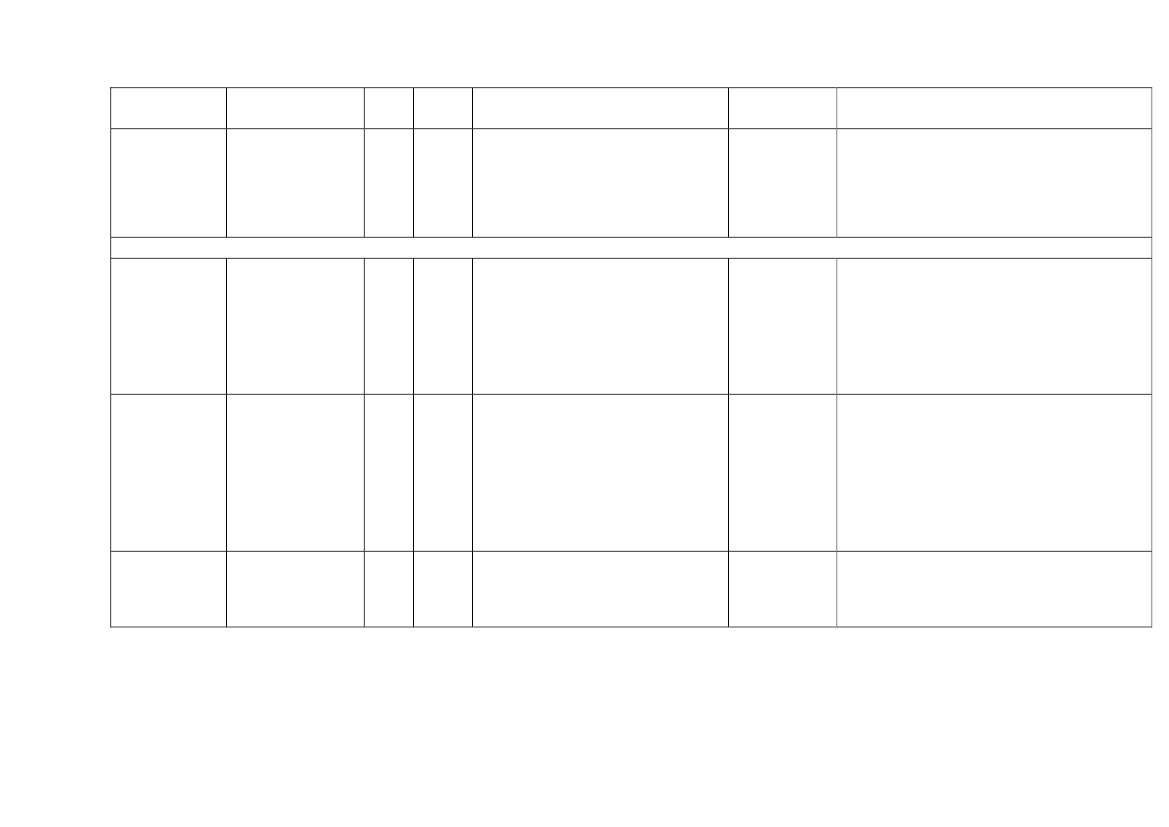
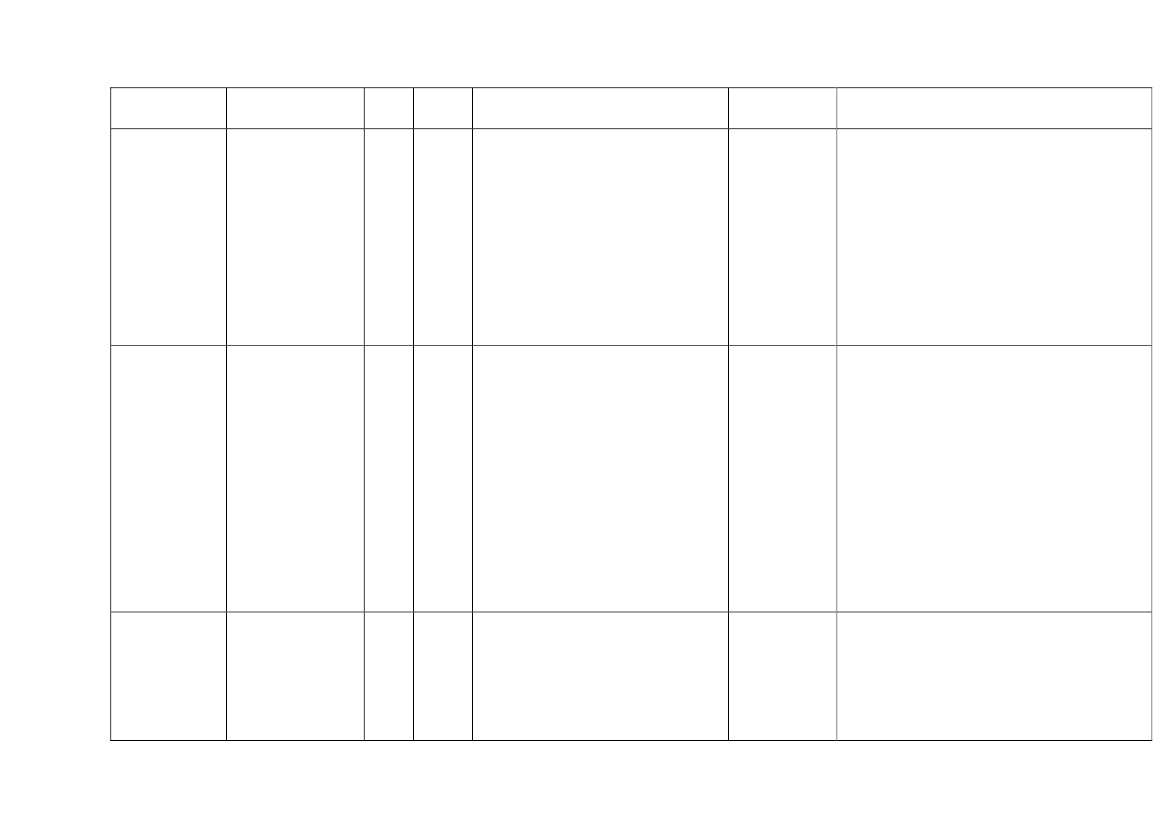
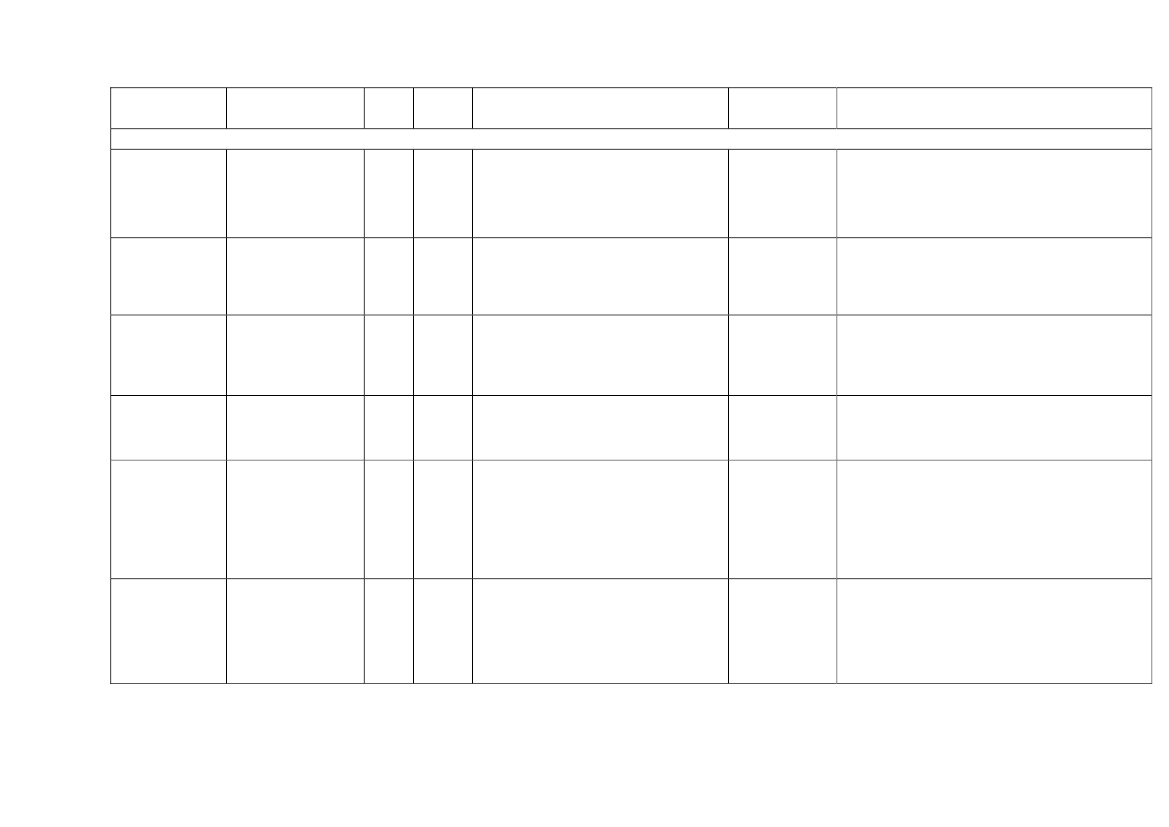
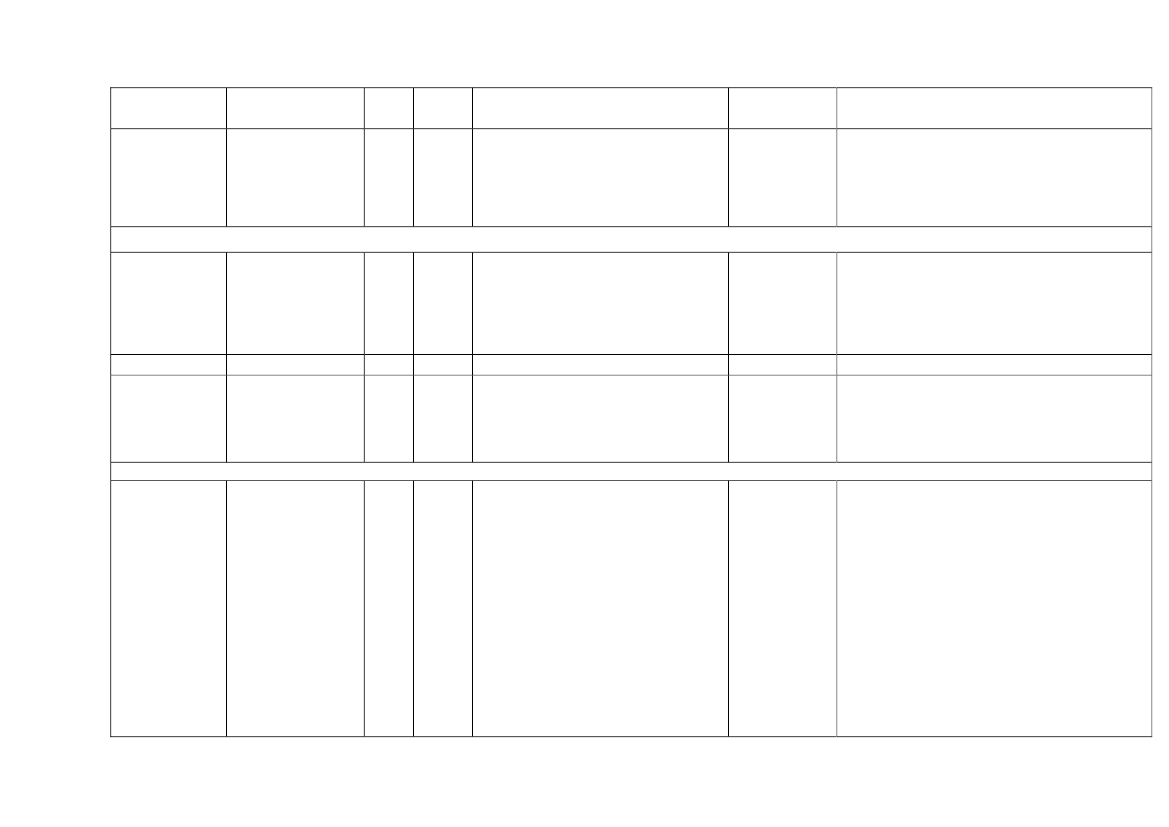
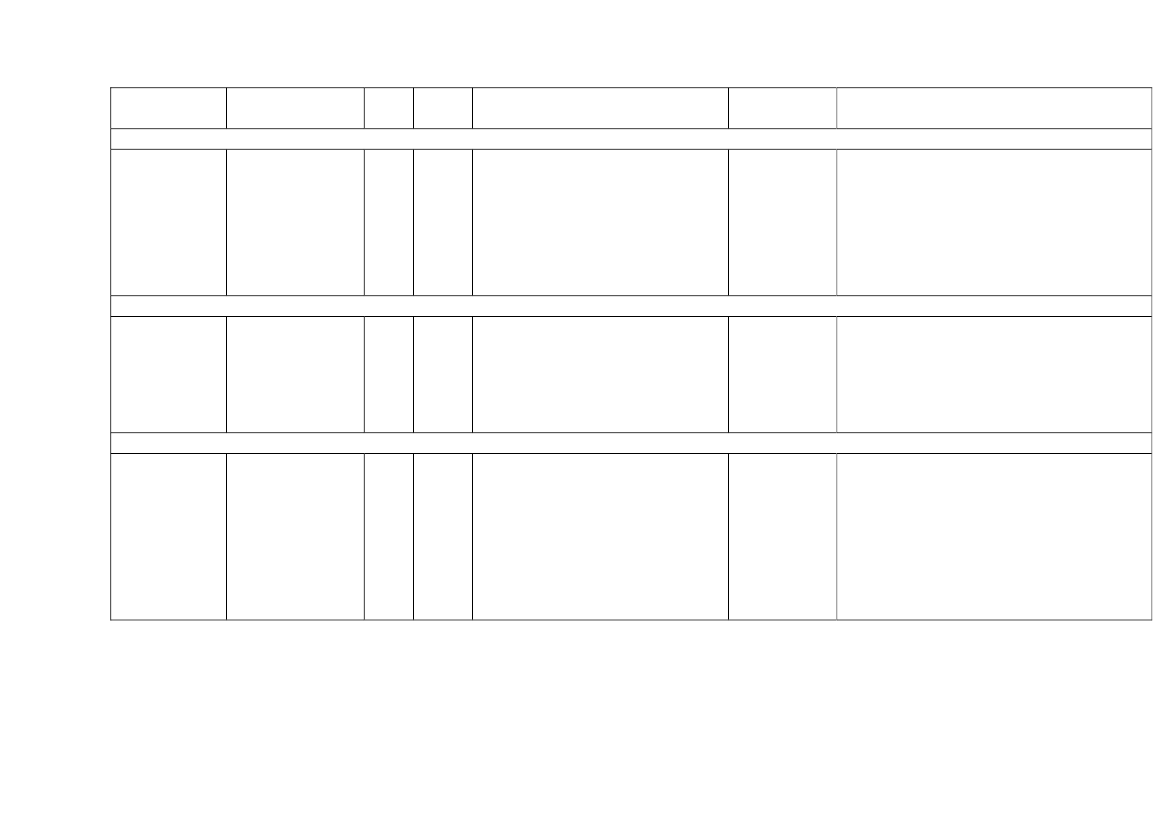
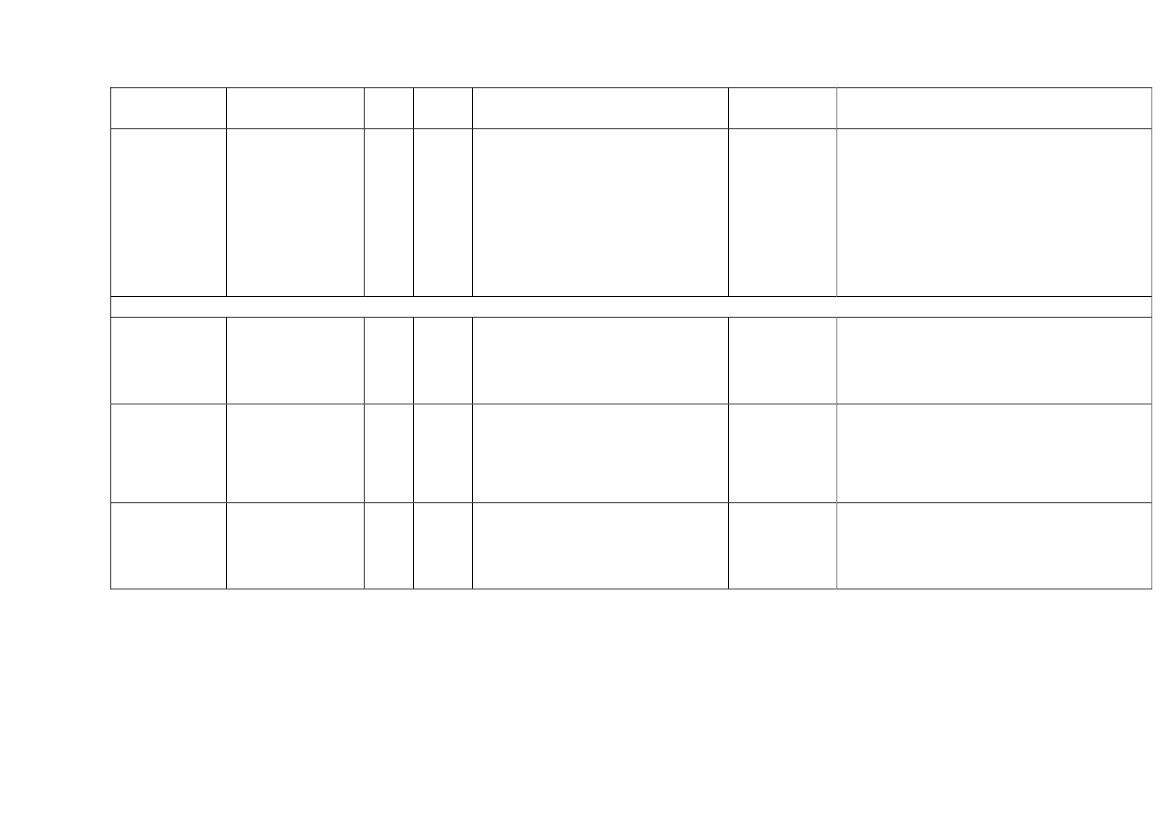
22toxicity, carcinogens and reproductive toxicants. The minimum information requirements forrepeated dose toxicity and reproductive toxicity are summarized in Table 1. It should be noted thatinterpretation of the testing requirements by the registrants in practice depends on a weight-of-evidence evaluation of existing data and may therefore be different to the minimum requirements aspresented here.Table 1. Repeated dose toxicity and reproductive toxicity testing minimum informationrequirements under REACH by tonnage level≥1t/yearNone≥ 10 t/year28-day repeateddose oral toxicitystudy in rodents(OECD TG 407)Screening forreproductive/developmental toxicity(OECD TGs 421 or422)≥ 100 t/year90-day repeateddose oral toxicitystudy in rodents(OECD TG 408)Prenataldevelopmenttoxicity study(OECD TG 414) inone species, and ifappropriate in asecond species≥ 1000 t/year90-day repeateddose oral toxicity study inrodents(OECD TG 408)Prenatal development toxicitystudy (OECD TG 414) in onespecies, normally in a secondspeciesTwo-generation reproductiontoxicity study (OECD TG 416)
Repeateddose toxicity
Reproductivetoxicity
None
5.1.1 Repeated dose toxicityBoth the 28- and the 90- day studies (OECD TG 407 and OECD TG 408, respectively) are includedin level 4 of the OECD Conceptual Framework, however only OECD TG 407 has been validated inrelation to identification of endocrine disrupters. The validation of OECD TG 407 in relation toendocrine endpoints showed that this assay is relatively insensitive and would only detect chemicalsthat are moderate and strong EDs for (anti)-estrogenicity and (anti)-androgenicity (e.g.ethinylestradiol and flutamide) (OECD GD 150). The assay did, however, detect EDs that wereweak and strong modulators of thyroid hormone-related effects (e.g. propylthiouracil and methyltestosterone). It may also detect steroidogenesis inhibition although only one (potent) chemical wasused in the validation study (OECD TG 407).The OECD TG 407 measures some parameters which are relevant to endocrine-mediated toxicitysuch as the weight and histopathology of the pituitary, adrenals, ovaries and ventral prostate. Someof the endpoints, particularly those related to the thyroid, are optional, and the lack of relevantendpoints is particularly striking for those most relevant to the testicular dysgenesis syndrome(Kortenkamp et al. 2012). In conclusion, there are major limitations for these studies in terms ofscreening for endocrine disrupting properties and these are mainly related to the fact that only adultanimals are exposed and the limited sensitivity of the gross endocrine endpoints.5.1.2 CarcinogenicityThere are no standard information requirements in relation to identification of carcinogenicproperties for substances produced or imported in quantities of less than 1000 tons per year. Acarcinogenicity study for substances produced or imported in quantities ≥ 1000 tons per year maybe required if the substance has a widespread dispersive use or there is evidence of frequent or long-
23term human exposure and the substance is classified as mutagen category 3 or there is evidencefrom the repeated dose toxicity study(ies) that the substance is able to induce hyperplasia and/orpre-neoplastic lesions.Some endpoints that are relevant in relation to hormonally mediated cancers are included in therepeated dose toxicity tests and may trigger a carcinogenicity study if information on use andhuman exposure warrant it. The limitations of standard repeated dose studies (OECD TG 407 andOECD TG 408) in terms of the timing of exposure and the sensitivity of the endpoint have alreadybeen mentioned above and this raise doubt over the likelihood that potential effects on hormonallymediated carcinogenesis will be detected on the basis of those tests (Kortenkamp et al. 2012).5.1.3 Reproductive toxicityThe minimum information requirements in relation to reproductive toxicity are summarised inTable 1.Neither the combined repeated dose toxicity/reproductive developmental toxicity screening tests(OECD TGs 421/422) nor the prenatal development toxicity study have yet been validated for thedetection of endocrine disrupters. In the prenatal development toxicity study (OECD TG 414),animals are exposed from implantation to two days before expected birth and in the combinedrepeated dose toxicity/reproductive toxicity screening studies animals are exposed from two weeksprior to mating to four days postnatally. Although these tests include exposure during pregnancy,the endpoints related to fertility and gestation maintenance are measured in the parent generation.Thus, a major limitation of these studies is that they do not include exposure during criticalwindows of development for those endpoints.In the prenatal development toxicity study (OECD TG 414), the foetuses are inspected for grossanomalies. However, important differences between humans and rodents concerning the timing ofbirth compared to developmental stage should be borne in mind. Rodents are compared to humansborn at a relatively immature stage and some parts of the sexual differentiation of the brain andreproductive organs that take place during the third trimester of human pregnancy occur after birthin the rat. This means that data from the prenatal development toxicity study have very limited usefor evaluating effects of EDs during the third trimester of human pregnancy.Gross evaluation of anogenital distance is generally used for sexing the offspring in reproductivetoxicity studies, because anogenital distance is normally twice as long in males compared tofemales. Thus major effects on male sexual differentiation induced by potent anti-androgens may bedetected as all offspring may display female-like anogenital distance (e.g. Hass et al. 2007).In conclusion, although these studies include endocrine relevant endpoints for fertility effects anddevelopmental effects, they have major limitations with regards to the endpoints related to fertilityas the exposure is not during critical windows of development and they also have very limitedsensitivity for detecting effects of EDs on sexual differentiation. Furthermore, the lower number ofanimals used (8-10 parental males and females) decrease their statistical power compared to e.g. thetwo-generation study and the extended one-generation study. These considerations raise uncertainty
24as to the ability of the current testing requirements to adequately screen for endocrine disruptingproperties at tonnage levels below 1000 tons per year (Kortenkamp et al. 2012).For chemicals with a supply tonnage level over 1000 tons per year, a two-generation study isgenerally required. This study includes exposure during sensitive time windows of development andassessment of a number of endpoints sensitive to endocrine disruption in the offspring. Results oftwo-generation reproduction toxicity studies (OECD TG 416) should nonetheless be interpretedwith caution: some endocrine sensitive endpoints were added only in 2001 as a result of an updateof the guideline. Further, some endpoints sensitive to endocrine disruption are not included in theupdated version of the two-generation reproduction study, such as nipple retention, anogenitaldistance at birth, and measurement of thyroid hormones. Thus, for the two-generation reproductiontoxicity study there are uncertainties with regard to the ability to adequately detect endocrinedisrupters.The new extended one-generation reproduction toxicity study (OECD TG 443) includes the abovementioned ED sensitive endpoints as well as assessment of neurodevelopment and immunotoxicity.Thus, the new EOGRT study (OECD TG 443) is preferable for detecting endocrine disruptionbecause it provides an evaluation of a number of endocrine endpoints in the juvenile and adult F1,which are not included in the 2-generation study (OECD TG 416) adopted in 2001(OECD GD 150).This test is also expected to have greater sensitivity than OECD TG 416 as it requires an increasednumber of pups to be examined. In summary, the exposure of the foetus (which is a sensitive life-stage for endocrine disruption effects), the long duration of dosing and the diversity of endpointsmeans that the extended one-generation study may be considered to be the most predictive test forED-mediated adverse effects via EATS modalities (OECD GD 150). Therefore, the use of theextended one-generation reproduction toxicity study (OECD TG 443) instead of the two-generationstudy would significantly enhance the ability for detection of endocrine disrupters at tonnage levelsabove 1000 tons per year.Delayed effects of developmental exposure to EDs that can manifest themselves only with ageingsuch as premature reproductive senescence are currently not included in any guideline study. SuchED effects are clearly severe, however, there is at present not sufficient scientific knowledge forevaluating whether effects observed earlier in life may protect also towards such late effects orwhether they may occur at lower doses than early effect.5.1.4 Sensitivity for finding a relevant threshold-like dose for EDs, using power analysisPower analysis can be used to calculate the minimum sample size required, in order to likely detectan effect of a given size. Power analysis can also be used to calculate the minimum effect size thatis likely to be detected in a study using a given sample size.A number of endpoints relevant for ED provide quantal data, i.e. they are results providing yes/noanswers, like for example data on malformations of reproductive organs, or fertility- and pregnancyindex. Assessment of quantal endpoints is generally expected to lead to a lower power thanassessment of continuous data (e.g. anogenital distance (AGD) or reproductive organ weights). Toexplore this, we have calculated the effect size needed for finding significant effects, i.e. p < 0.05,
25for yes/no endpoints and continuous endpoints. The methods and results from these calculations aredescribed in Appendix 3, whereas the next section will only provide the conclusions.To illustrate the importance of sample size, the power calculations were done for studies usingeither 8 or 20 litters per group, which are the group sizes required in the OECD TG 421/422(Reproduction/Developmental toxicity Screening study) and in the OECD TG 416 and OECD TG443(two- generation and extended one generation study), respectively. Overall, the resultsillustrated that the effect size for a quantal effect had to be 25-37% in studies with 20 litters pergroup, and even higher (50-75%) with only 8 litters per group. This implies that the sensitivity fordetecting quantal effects is very low and that effect sizes of human relevance may be present at theNOAEL.For continuous endpoints the statistical power for detecting significant effects depends on the groupsize, and on the coefficient of variation (CV) in the control group. For AGD data, the CV isnormally around 5- 7% and the calculations performed in appendix 2 show that in studies with 20animals per group, an effects size of ca. 4-7% will in most cases be statistically significant, whereasthe effect size has to be 7-11% if only 8 litters per group are studied. So for continuous data, effectsizes needed for detecting significant effects with 8 animals per group are approximately 1.6-1.8times higher than when 20 animals per group are used.Continuous effect data are generally expected to be more sensitive than quantal data and this wasalso found by the present calculations, as the effect sizes needed for continuous data ranged from 4-18%, whereas the effect sizes for quantal data were found to range from 25-75%. In spite of theincreased sensitivity of continuous data compared to quantal data, effect sizes of human relevancemay also be present at the NOAEL for continuous data.5.2 ConclusionsThe current information requirements in REACH are not designed for the identification ofendocrine disrupters, although certain endpoints and assays may give some indication of endocrinedisrupting effects. It is, however, evident that important endpoints needed for the detection of EDeffects are not included. Especially, important effects after exposure that cover windows ofsusceptibility during development are not assessed. This raises major uncertainty as to the ability ofthe current testing requirements to adequately detect EDs at tonnage levels below 1000 tons peryear. A two-generation reproduction toxicity study is generally required for chemicals with a supplytonnage level above 1000 tons per year and this study includes exposure during sensitive timewindows of development and assessment of a number of endpoints sensitive to endocrine disruptionin the offspring. However, some endocrine sensitive endpoints were added only in 2001 as a resultof an update of the guideline and others are not included in the updated version of the two-generation reproduction study, such as nipple retention, anogenital distance at birth, andmeasurement of thyroid hormones. Thus, for the two-generation reproduction toxicity study thereare also uncertainties with regard to the ability to adequately detect endocrine disrupters.
26The new extended one-generation reproduction toxicity study (OECD TG 443) includes the abovementioned ED sensitive endpoints. The exposure of the foetus (which is a sensitive life-stage forendocrine disruption effects), the long duration of dosing and the diversity of endpoints means thatthe extended one-generation study may be considered to be the most predictive test for ED-mediated adverse effects via EATS modalities (OECD GD 150). Therefore, using the extended one-generation study instead of the two-generation study would significantly enhance the ability fordetection of endocrine disrupters at tonnage levels above 1000 tons per year.Power calculations for studies using 8 or 20 litters per group, which are the group sizes required inthe OECD TG 421/422 (Reproductive Screening study) and in the OECD TG 416 and OECD TG443(two- and extended one generation study) illustrated that the effect size needed for detection ofquantal effects had to be 25-37% with 20 litters per group and 50-75% with 8 litters per group. Thisclearly shows that the sensitivity for detecting quantal effects is very low and that effects sizes ofhuman relevance may be present at the NOAEL. The effect sizes needed for continuous data rangedfrom 4-18%, so in spite of the increased sensitivity of continuous data compared to quantal data,effect sizes of human relevance may also be present at the NOAEL for continuous data.
6. Are EDs of particular concern?The European Commission published in the beginning of 2012 a report on "State of the artassessment of endocrine disruptors", where it is indicated that EDs are of similar concern as CMRs(carcinogens, mutagens, reproductive toxicants) and PBTs. The arguments for this include that EDsinduce irreversible and very severe effects and that exposure during sensitive windows ofdevelopment can lead to occurrence of such effects also later in life.Many endocrine disruptors are already or can be identified as carcinogens or reproductive toxicantsdue to the inherent endocrine disrupting properties. A common characteristic for CMRs is thateffects may often occur with a time lag of several years after the exposure.The majority of the effects potentially related to human exposure to EDs during developmentbecome manifest later in life, e.g. behavioural effects in children or adults, alterations of pubertytiming, low sperm quality, decreased fertility, increased risk for cancer in mammary tissue, prostateand testes, endometriosis and effects on menopause in women. This reflects that exposure duringearly development can lead to irreversible developmental programming affecting the health for therest of the individuals life time. Thus, there may be a time lag of many years or several decadesfrom regulatory decisions on risk reduction are taken, to the time when this risk reduction will beachieved and this is of particular concern when the regulation aims for reduction of risks tochemicals causing severe and delayed effects.A common reason for considering both PBTs and vPvBs as substances of very high concern isexpressed by the P, i.e. that the substances are persistent. A characteristic for both persistent andbioaccumulative substances is that exposure to these substances will occur long time after the initial
27source of exposure has ceased. This means that there can be a time lag of many years or decadesfrom regulatory decisions on risk reduction are implemented to the time when exposure to thesechemicals diminishes and it is therefore difficult to control the risk. With regard to persistent andbioaccumulating chemicals that are toxic due to endocrine disrupting properties, fat-soluble,persistent EDs are accumulated in the body fat and humans will be exposed for a long time after theinitial source of exposure to the substance has ceased. The consequences of long-term continuedexposure to bioaccumulated EDs for the complex functioning of the endogenous hormonal systemare largely unknown.In conclusion, EDs are evaluated as being of particular concern, because exposure during sensitivetime windows of development may cause irreversible developmental programming effects leadingto severe health effects manifested late in life, and also because the consequences of long-termcontinued exposure on the complex hormonal system are largely unknown.
7. Summary, conclusions and recommendationsThe aim of this report is, from a scientific point of view, to discuss the topics expected to berelevant for the REACH review on EDs, i.e.:- Thresholds or non-threshold assumption for ED effects- Considerations concerning non-monotonic dose-response (NMDR)- Uncertainties of the currently regulatory test methods with regard to determination ofpossible thresholds for EDs- Whether there is particular concern for EDs.The presence of thresholds can never be confirmed or rejected by experimental data, because allmethods for measuring effects have a limit of detection below which effects cannot be observed.Thus evaluations on whether effects of EDs should be assumed to exhibit a threshold or not have tobe based on a combination of biological plausibility and experimental observations. A generalargument for assuming no biological threshold for EDCs is that because low doses of endogenoushormones are present and fluctuating, small additions (or subtractions) to their actions will have asignificant impact. The validity of assuming no biological threshold for EDs is supported by thevery important organizing role of hormones during development at a time point where thehomeostatic control is not effective or not developed yet. Also, experimental data indicate non-thresholded dose-response for some endpoints for adverse effects on sexual differentiation such asanogenital distance and nipple retention at the dose levels studied so far. It is therefore concludedbased on a combination of biological plausibility and experimental observations that an assumptionof no threshold appears more valid for the effects of EDs during development than an assumption ofa threshold.Regardless of ED mode of action, it is uncertain whether or not there is a threshold for EDs. ForEDs, where the MoA (Mode of Action) directly involve the receptor, the interaction with thereceptor is likely to have no threshold. For EDs affecting the hormone levels, the response pattern
28may appear threshold-like, because multiple pathways converge before seeing the final responseand some of these pathways may have a threshold.Irrespective of threshold or non-threshold, the dose response curves of EDs seem generally to bebest described as sigmoid curves, i.e. the effect decreases asymptotically with dose towards zero butdoes not become zero, as supported by several types of experimental data. Such curves, however,have a “threshold-like” appearance, but a threshold cannot be inferred from the shape of the dose-response curves. However, a benchmark approach may be used for estimating a human exposurelevel with very low risk.There are several mechanisms that illustrate how hormones and EDs may cause NMDRs due to thefunction of the endocrine system. These mechanisms include receptor selectivity, receptor down-regulation and desensitization, receptor competition, and endocrine negative feedback loops.NMDR for EDs exists and have been shown and used in human endocrinology as a basic principlebehind the pharmaceutical treatment of severe diseases. Also, NMDR has been shown for manydifferent ED-mediatedin vitroandin vivoeffects including binding to steroid hormone receptorsand adverse effects on reproductive organ weights (prostate and testis), nipple retention and sexualmaturation. In many of the cases the observed NMDR is likely to directly reflect the way theendocrine system works. In other cases, the NMDR may reflect that the substance has multiple EDmodes of action operating simultaneously, but with different dose-response curves. As detailedmechanistic knowledge is limited for most EDs it is often difficult to evaluate the MoA behindNMDR.The current information requirements in REACH are not designed for the identification ofendocrine disrupters, although certain endpoints and assays may give some indication of endocrinedisrupting effects. It is, however, evident that important endpoints needed for the detection of EDeffects are not included. Especially, important effects after exposure that cover windows ofsusceptibility during development are not assessed. This raises major uncertainty as to the ability ofthe current testing requirements to adequately screen for endocrine disrupting properties at tonnagelevels below 1000 tons per year. A two-generation reproduction toxicity study is generally requiredfor chemicals with a supply tonnage level above 1000 tons per year and this study includesexposure during sensitive windows of development and assessment of a number of endpointssensitive to endocrine disruption in the offspring. However, some endocrine sensitive endpointswere added only in 2001 as a result of an update of the guideline and others are not included in theupdated version of the two-generation reproduction study, such as nipple retention, anogenitaldistance at birth, and measurement of thyroid hormones. Thus, for the two-generation reproductiontoxicity study there are also uncertainties with regard to the ability to adequately detect endocrinedisrupters.The new extended one-generation reproduction toxicity study (OECD TG 443) includes the abovementioned ED sensitive endpoints. The exposure of the foetus (which is a sensitive life-stage forendocrine disruption effects), the long duration of dosing and the diversity of endpoints means thatthe extended one-generation study may be considered to be the most predictive test for ED-mediated adverse effects via EATS modalities (OECD GD 150). Therefore, using the extended
29one-generation study instead of the two-generation study would significantly enhance the ability fordetection of endocrine disrupters at tonnage levels above 1000 tons per year.Power calculations for studies using 8 or 20 litters per group, which are the group sizes required inthe OECD TG 421/422 (Reproductive Screening study) and in the OECD TG 416 and OECD TG443(two- and extended one generation study) illustrated that the effect size needed for detection ofquantal effects have to be 25-37% with 20 litters per group and 50-75% with 8 litters per group.This clearly shows that the sensitivity for detecting quantal effects is very low and that effects sizesof human relevance may be present at the NOAEL. The effect sizes needed for continuous datarange from 4-18%, so in spite of the increased sensitivity of continuous data compared to quantaldata, effect sizes of human relevance may also be present at the NOAEL for continuous data.The majority of the effects potentially related to human exposure to EDs during developmentbecome manifest later in life, e.g. behavioural effects in children or adults, alterations of pubertytiming, low sperm quality, decreased fertility, increased risk for cancer in mammary tissue, prostateand testes, endometriosis and effects on menopause in women. This reflects that exposure duringearly development can lead to irreversible developmental programming affecting the health for therest of the individuals life time and possibly also future generations. Thus, there may be a time lagof many years or several decades from regulatory decisions on risk reduction are taken, to the timewhen this risk reduction will be achieved and this is of particular concern when the regulation aimsfor reduction of risks to chemicals causing severe and delayed effects. In conclusion, EDs areevaluated as being of particular concern due to the ability for causing severe and irreversible effectsthat may possibly also be manifested through next generations, because exposure are especiallyproblematic during sensitive time windows of development, and because the consequences of long-term continued exposure on the complex hormonal system are largely unknown.Overall, it concluded that there are major uncertainties in relation to the detection of safe levels forhuman exposure to EDs. These uncertainties include that:--During development an assumption of no threshold appears more valid than an assumptionof a threshold.For EDs, where the MoA directly involve the receptor, the interaction with the receptor islikely to have no threshold. For EDs affecting the hormone levels, there may be a threshold,if the substances affect the hormone levels via a mechanism where there is a threshold.NMDR for EDs exists and this knowledge is used in human endocrinology as a basicprinciple behind the pharmaceutical treatment of severe diseases. Also, NMDR has beenshown for many ED-mediatedin vitroandin vivoeffects including binding to steroidhormone receptors and adverse effects and this can directly reflect the way the endocrinesystem works.There are major limitations as to the ability of the current testing requirements to adequatelyscreen for endocrine disrupting properties and effect sizes of human relevance may bepresent at the NOAEL.
-
-
30-Delayed effects of developmental exposure to EDs that can manifest themselves only withageing such as premature reproductive senescence are currently not included in anyguideline study.EDs are evaluated as being of particular concern, because exposure during sensitive timewindows of development may cause irreversible developmental programming effectsleading to severe health effects manifested late in life, and also because the consequences oflong-term continued exposure on the complex hormonal system are largely unknown.
-
Based on these conclusions, we recommend that:- A sufficient regulatory testing scheme should be developed for detection of EDs.Assessment of adverse ED effects as well as investigations of MoA are relevant as both areimportant for evaluating whether a substance is an ED- Enhancement of the Reproduction/developmental toxicity Screening studies (OECD TG421/422) and the Prenatal developmental toxicity study (OECD TG 414) with regard todetection of ED effects should be considered- The new extended one-generation reproduction toxicity study (OECD TG 443) shouldreplace the two-generation reproduction toxicity study (OECD TG 416), as this wouldsignificantly enhance the ability to identify endocrine disrupting substances- The scientific knowledge needed for evaluating whether ED effects observed early in lifealso protects towards delayed effects that becomes manifest only with ageing such aspremature reproductive senescence should be improved- Effects seen at low doses, but not at higher doses of EDs should be carefully evaluated andinterpreted, as EDs may cause NMDR- The number of dose levels in experimental studies should be increased to better characterizethe dose-response and increase the possibility for detection of NMDR- A benchmark dose (BMD) approach where both effect size and severity is included shouldbe used when estimating human risk instead of a NOAEL approach. Using the BMDapproach, poor data quality will lead to a lower BMD and better data, with their reduceddegree of uncertainty, are “rewarded” with higher BMDs whereas poor data quality usuallyresults in higher NOAELs. However, sufficient dose-response data for a BMD approachmay in many cases not be available and NMDR may not be detected with current regulatorytesting where only three dose levels are required.
31
8. ReferencesAbt E, Rodricks JV, Levy JI, Zeise L, Burke TA. 2010 Science and decisions: advancing riskassessment. Risk Anal. 2010 Jul;30(7):1028-36. doi: 10.1111/j.1539-6924.2010.01426.x. Epub2010 May 20.Alm, H.; Kultima, K.; Scholz, B.; Nilsson, A.; Andren, P. E.; Fex- Svenningsen, A.; Dencker, L.;Stigson, M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal proteinexpression in the neonatal mouse cerebral cortex. Neurotoxicology 2008, 29(4), 628–37.Almstrup K, Fernandez MF, Petersen JH, Olea N, Skakkebaek NE, Leffers H 2002 Dual effects ofphytoestrogens result in U-shaped dose-response curves. Environ Health Perspect 110:743–748Andrade AJM, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following inutero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology 2006;227:185-192.Ashby J, Tinwell H, Odum J, Lefevre P 2004 Natural vari- ability and the influence of concurrentcontrol values on the detection and interpretation of low-dose or weak endocrine toxicities. EnvironHealth Perspect 112:847– 853Asp V, Ullerås E, Lindström V, Bergström U, Oskarsson A, Brandt I 2010 Biphasic hormonalresponses to the adrenocorticolytic DDT metabolite 3-methylsulfonyl-DDE in human cells. ToxicolAppl Pharmacol 242:281–289Blair, R.M., Fang, H., Gaylor, D., Sheehan, D.M., 2001. Threshold analysis of selected dose–response data for endrocrine disruptors. Acta Path. Microbiol. Immunol. Scand. 109, 198–208.Boettcher M, Kosmehl T, Braunbeck T 2011 Low-dose effects and biphasic effect profiles: Istrenbolone a genotoxicant? Mutat Res 723:152–157Boobis AR, Datson GP, Preston RJ, Olin SS. 2009. Application of Key Events Analysis toChemical Carcinogens and Noncarcinogens. Critical Reviews in Food Science and Nutrition,49:690-707.Campagna C, Ayotte P, Sirard MA, Arsenault G, Laforest JP, Bailey JL 2007 Effect of anenvironmentally relevant metabolized organochlorine mixture on porcine cumulus- oocytecomplexes. Reprod Toxicol 23:145–152Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB & Hass U.(2010) Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects inmale rats. Reprod Toxicol 30, 313–321.
32
Conolly RB, Lutz WK 2004 Nonmonotonic dose-response relationships: mechanistic basis, kineticmodeling, and im- plications for risk assessment. Toxicol Sci 77:151–157Ebersolt C, Premont J, Prochiantz A, Perez M, Bockaert J. Inhibition of brain adenylate cyclase byA1 adenosine receptors: pharmacological characteristics and locations. Brain Res. 1983 May9;267(1):123-9.European Chemicals Agency, 2007. Guidance for the preparation of an Annex XV dossier on theidentification of substances of very high concern. ECHA. Helsinki, Finland).http://echa.europa.eu/documents/10162/13638/svhc_en.pdf .Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, Sealfon SC, Bernard DJ, HardyMP. Biphasic Effects of Postnatal Exposure to Diethylhexylphthalate on the Timing of Puberty inMale Rats. J Androl 2007;28:513-520.Gray L, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicidevinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health 1999;15:48-64.Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB,Kortenkamp A 2007 Combined exposure to anti-androgens exacerbates disruption of sexualdifferentiation in the rat. Environmental Health Perspectives 115 Suppl 1:122-128Jacobsen PR, Axelstad M, Boberg J, Isling LK, Christiansen S, Mandrup KR, Berthelsen LO,Vinggaard AM, Hass U. Persistent developmental toxicity in rat offspring after low dose exposureto a mixture of endocrine disrupting pesticides. Reprod Toxicol. 2012 Sep;34(2):237-50. Epub 2012Jun 4.Jeng YJ, Kochukov MY, Watson CS 2009 Membrane estrogen receptor-alpha-mediatednongenomic actions of phytoestrogens in GH3/B6/F10 pituitary tumor cells. J Mol Signal 4:2Kohn MC, Melnick RL 2002 Biochemical origins of the non-monotonic receptor-mediated dose-response. J Mol Endocrinol 29:113–123Kortenkamp A, Martin O, Faust M, Evans R, McKinlay R, Orton F, Rosivatz E. (2012). State of theArt Assessment of Endocrine Disrupters. Final Report. [Online] Available at:http://ec.europa.eu/environment/endocrine/documents/4_SOTA%20EDC%20Final%20Report%20V3%206%20Feb%2012.pdfLeung LY, Kwong AK, Man AK, Woo NY 2008 Direct actions of cortisol, thyroxine and growthhormone on IGF-I mRNA expression in sea bream hepatocytes. Comp Biochem Physiol A MolIntegr Physiol 151:705–710
33Maness SC, McDonnell DP, Gaido KW(1998) Inhibition of androgen receptor-dependenttranscriptional activity by DDT isomers and methoxychlor in HepG2 human hepatoma cells.Toxicol Appl Pharmacol 151:135–142.Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Gallo M, Reuhl K, Ho S, Brown T,Moore J, Leakey J, Haseman J, Kohn M (2002) Summary of the National Toxicology Program’sReport of the Endocrine Disruptors Low-Dose Peer Review. Env Health Perspect 110: 427-431.Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, et al. 2007.Dysgenesis and histological changes of genitals and perturbations of gene expression in male ratsafter in utero exposure to antiandrogen mixtures. Toxicol Sci 98:87–98.Moore, KL 1983. Before we are born: Basic Embryology and Birth DefectsSecond edition Saunders (ISBN 10: 0721610242 / ISBN 13: 9780721610245 )Naciff, J. M.,Hess, K. A., Overmann, G. J., Torontali, S. M.,Carr, G. J., Tiesman, J. P., Foertsch, L.M., Richardson, B. D., Martinez, J. E. and Daston, G. P. (2005b). Gene expression changes inducedin the testis by transplacental exposure to high and low doses of 17-alpha-ethynyl estradiol,genistein, or bisphenol A. Toxicol. Sci. 86:396–416.National Toxicology Program 2001 National Toxicology Program’s report of the endocrinedisruptors low dose peer review. Research Triangle Park, NC: National Institute of EnvironmentalHealth SciencesOECD test guidelines (human health): http://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788OECD 2012: Guidance Document on Standardised Test Guidelines for Evaluating Chemicals forEndocrine Disruption. Series on Testing and Assessment No. 150, ENV/JM/MONO(2012)22, 24-Aug-2012;http://www.oecd.org/env/ehs/testing/seriesontestingandassessmentpublicationsbynumber.htmOhlsson A, Cedergreen N, Oskarsson A, Ullerås E 2010 Mixture effects of imidazole fungicides oncortisol and aldosterone secretion in human adrenocortical H295R cells. Toxicology 275:21–28Putz O, Schwartz CB, Kim S, LeBlanc GA, Cooper RL, Prins GS 2001 Neonatal low- and high-dose exposure to estradiol benzoate in the male rat. I. Effects on the prostate gland.Biol Reprod65:1496 –1505Ralph JL, Orgebin-Crist MC, Lareyre JJ, Nelson CC 2003 Disruption of androgen regulation in theprostate by the environmental contaminant hexachlorobenzene. Environ Health Perspect111:461–466
34Scholze M, Kortenkamp A. 2007 Statistical power considerations show the endocrine disruptorlow-dose issue in a new light.Environ Health Perspect. 2007 Dec;115 Suppl 1:84-90. doi: 10.1289/ehp.9364.Sheehan DM et al. 1999. No threshold dose for estradiol induced sex reversal of turtle embryos:how little is too much? Environ Health Perspect 107: 155-159.Sheehan DM. No-threshold dose-response curves for nongenotoxic chemicals: findings andapplications for risk assessment.Environ Res. 2006 Jan;100(1):93-9. Epub 2005 Oct 26.Siler-Khodr TM, Fetal Hormones page307-317 in Encyclopedia of Reproduction, 2ndVolume Set,1998, Academic PressSlob W. 1999. Thresholds in Toxicology and Risk Assessment. International Journal of Toxicology18:259-268; Scholze M and Kortenkamp A. 2007. Statistical power considerations show theendocrine disrupter low dose issue in a new light. Environ Health Perspect 115 Suppl 1: 84-90.Somjen D, Kohen F, Jaffe A, Amir-Zaltsman Y, Knoll E, Stern N 1998 Effects of gonadal steroidsand their antag- onists on DNA synthesis in human vascular cells. Hyper- tension32:39 – 45Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs, Jr DR., Lee D-H, Shioda T, Soto AM,vom Saal FS, Welshons WV, Zoeller RT, and Myers JP. Hormones and Endocrine-DisruptingChemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocrine Reviews, June 2012,33(3):378-455vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK,Parmigiani S, Welshons WV 1997 Prostate enlargement in mice due to fetal exposure to low dosesof estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA94:2056 –2061Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom SaalFS. Endocrine-disrupting chemicals and public health protection: a statement of principles from TheEndocrine Society Endocrinology. 2012 Sep;153(9):4097-110. doi: 10.1210/en.2012-1422. Epub2012 Jun 25
35
Appendix 1This appendix gives some more details on the 80 evaluatedin vitroexamples allocated into four groups. Almost half of the examples (45%)could not according to our definition be regarded as showing a true non-monotonic dose-response, as the NMDR was evaluated as due tocytotoxicity. Furthermore, some examples were evaluated as “false NMDR”, because of e.g. testing of mixtures or limitations in the studydesign. The remaining examples were evaluated to either show evidence for NMDR (16%) or a dose-response that may or may not be dueto NMDR of EDs (17%).
36Chemicals bychemical classNatural hormones17R-EstradiolNonmonotonic effectCell typeRefs.Evaluation
5a-Dihydrotestosterone5a-AndrostenedioneCorticosteroneInsulinProgesteroneProlactinhCGT3GHPharmaceutical hormonesDESEthinyl estradiolR1881 (synthetic androgen)TrenbolonePlasticsBPA
Cell numberDopamine uptakepERK levels, prolactin releaseR-Hexosaminidase releaseCell numberProduction of L-PGDS, a sleep-promoting substanceCell numberCell number, kinase activityCell numberMitochrondrial oxidation, calcium fluxMarkers of apoptosis (in absence of glucose)Cell numberTestosterone releaseTestosterone releaseRate of protein phosphorylationLPLmRNA expressionIGF-IexpressionCell numberProlactin releaseCXCL12 secretionCell numberInduction of micronucleiCell numberDopamine effluxpERK levels, intracellular CaCell numberNumber of coloniesNumber of colonies2+
MCF7 breast cancer cellsFetal hypothalamic cells (primary)GH3/B6/F10 pituitary cellsHMC-1 mast cellsVascular smooth muscle cellsU251 glioma cellsLNCaP-FGC prostate cancer cellsVascular smooth muscle cellsLNCaP-FGC prostate cancer cellsCortical neurons (primary)Pancreatic R-cells (primary)LNCaP-FGC prostate cancer cellsAdult rat testicular cells (primary)Adult rat testicular cells (primary)Cerebral cortex cells (primary, synaptosomes)White adipocytes (rat primary)Hepatocytes (primary cultures from silver sea bream)MCF7 breast cancer cellsGH3/B6/F10 pituitary cellsMCF7 breast cancer cells, T47D breast cancer cellsLNCaP-FGC cellsRTL-W1 fish liver cellsMCF7 breast cancer cellsPC12 rat tumor cells
135, 71671741, 718, 71972072172249972149972372449972572572672772871641729499730135, 7164041, 718731732732
CytotoxMaybeNMDRCytotox?FalseCytotoxCytotoxFalseCytotoxCytotoxCytotoxCytotoxCytotoxCytotoxNMDRFalseFalseCytotoxNMDRMaybeCytotoxNMDRCytotoxNMDRNMDRFalseMaybeMaybe
DEHPDi-n-octyl phthalate
changes, prolactin release GH3/B6/F10 pituitary cellsLNCaP prostate cancer cellsEscherichia coliandB. subtilisbacteriaE. coliandB. subtilisbacteria
37Chemicals bychemical classDetergents, surfactantsOctylphenolNonmonotonic effectCell typeRefs.Evaluation
PropylphenolNonylphenol
Cell numberDopamine uptakepERK levelsHCG-stimulated testosterone levelspERK levelspERK levels, prolactin releaseR-Hexosaminidase releaseCell number
MCF7 breast cancer cellsFetal hypothalamic cells (primary)GH3/B6/F10 pituitary cellsLeydig cells (primary)GH3/B6/F10 pituitary cellsGH3/B6/F10 pituitary cellsHMC-1 mast cellsMCF7 breast cancer cellsP19 embryonic carcinoma cellsP19 embryonic carcinoma cellsPieces of goldfish testesPieces of goldfish testesPieces of goldfish testesPostvitellogenic follicles (isolated from catfish)Human endometrial endothelial cellsCaco-2BBe colon adenocarcinoma cellsT47D breast cancer cellsPC3 prostate cancer cellsGH3/B6/F10 pituitary cellsGH3/B6/F10 pituitary cellsGH3/B6/F10 pituitary cellsMCF7 breast cancer cellsLoVo colon cancer cellsHuman umbilical vein endothelial cellsGH3/B6/F10 pituitary cellsMCF7 breast cancer cellsMCF7 breast cancer cellsMCF7 breast cancer cellsYeast bioassayH295R adrenocortical carcinoma cellsSCC-25 oral squamous carcinoma cells
71671771873371841, 718720135734, 735734736736736737738739729740719719719135741742719743743744745746747
CytotoxMaybeNMDRNMDRNMDRNMDRCytotoxCytotoxMaybeMaybeCytotoxCytotoxCytotoxCytotoxMaybeCytotoxMaybeCytotoxNMDRNMDRNMDRCytotoxMaybeNMDRNMDRMaybeMaybeMaybeMaybeCytotoxMaybe
PAHPhenanthreneAll-trans retinoic acid activityBenz(a)acridineAll-trans retinoic acid activityNaphthalenehCG-stimulated testosteroneB-naphthoflavonehCG-stimulated testosteroneRetenehCG-stimulated testosteroneHeavy metalsLeadEstrogen, testosterone, and cortisol levelsCadmiumExpression of angiogenesis genesPhytoestrogens and natural antioxidantsGenisteinCell numberCXCL12 secretion, cell numberCell number, cell invasion, MMP-9 activityCoumesterolDaidezinpJNK levels, Ca fluxProlactin release, pERK levelsProlactin release, pERK levelsCell numberCell numberExpression of angiogenesis genes2+2+
ResveratrolTrans-resveratrolArtelastochromeneCarpelastofuranBiochanin ALicoflavone CQuercetin
pERK levels, Ca fluxCell numberCell numberInduction of estrogen-sensitive genesInduction of estrogen-sensitive genesAromatase activityCell number
38Chemicals bychemical classNonmonotonic effectCell typeRefs.Evaluation
DioxinTCDDCell number, gene expressionPCBPCB-74Cell viability, GnRH peptide levelsPCB-118Cell viability, GnRH peptide levelsAroclor 1242 (PCB mixture)β-Hexosaminidase releasePOP mixtureApoptosis of cumulus cellsHerbicidesGlyphosphate-herbicide (Round-Up) Cell death, aromatase activity, ERβ activityAtrazineCell numberInsecticidesEndosulfanCell numberβ -Hexosaminidase releaseATPase activity of P-glycoproteinDiazinonCell numberDieldrinβ -Hexosaminidase releaseDDTCell numberDDEβ-Hexosaminidase releaseProlactin releaseCortisol and aldosterone release, steroidogenic genes3-Methylsulfonyl-DDEFungicidesHexachlorobenzeneTranscriptional activity in the presence of DHTProchlorazAldosterone, progesterone, and corticosterone levels;expression of steroidogenic genesKetoconazoleAldosterone secretionFungicide mixturesAldosterone secretionPBDEPBDE-49Activation of ryanodine receptor 1PBDE-99Expression of GAP43
M13SV1 breast cellsGT1-7 hypothalamic cellsGT1-7 hypothalamic cellsHMC-1 mast cellsOocyte-cumulus complexes (primary, from pigs)HepG2 liver cellsIEC-6 intestinal cellsIEC-6 intestinal cellsHMC-1 mast cellsCHO cell extractsIEC-6 intestinal cellsHMC-1 mast cellsMCF7 breast cancer cellsHMC-1 mast cellsGH3/B6/F10 pituitary cellsH295R adrenocortical carcinoma cellsPC3 prostate cancer cellsH295R adrenocortical cellsH295R adrenocortical cellsH295R adrenocortical cellsHEK293 cell (membranes)Cerebral cortex cells (primary)
74874974972075075175275272075375272014472041754755756757757758759
MaybeCytotoxCytotoxCytotoxFalseCytotoxFalseFalseCytotoxMaybeFalseCytotoxNot evaluatedCytotoxNMDRCytotoxCytotoxCytotox?FalseFalseCytotoxCytotox
39
Appendix 2This appendix gives some more details on the 34 evaluated examples (invivo)allocated into the three groups. The majority of the studies,i.e. 22, were evaluated to give some evidence for NMDR (Group 2) and 5 studies showed clear evidence (Group 1). Poor or no evidence forNMDR was found for 7 of the studies (Group 3).
40
Chemical classNatural hormones17-Estradiol
NMDR effect;Organ/sex/speciesMorphologicalparameters;Mammarygland/female/mice
Refs.
Group
MoA and other relevant text (mainly asdescribed in the paper)The induction of estrogen-target genes inthe mammary gland was monotonic inboth strains. This type of dose–responsecurve suggests that estrogens can evokedifferent effects dependingon the different doses at which theseeffects were tested. The combined effect ofthese variable responses is reflected in theoverall cell number. Similarly, in themammary gland, estrogens induceproliferation, manifested as ductal growth,while concurrently inducing apoptosis,manifested as lumen formation.Potential mechanisms mediating adecrease in prostate weight in response tosupraphysiological doses of estrogeninclude receptor down-regulation andthe capacity for estradiol to bind toreceptors for other steroids, such asandrogen receptors, resulting inantagonistic effects mediated via otherreceptor systems.
No per group;no. doses5 per group, 2strains; 8 groups
Further details and remarks
138,541
1
Dose-related monotonic increase in uterine weight,non-monotonic (inverse U-shaped) dose-response fornumber of terminal end buds, ductal extension andductal area in one of the mice strains. It was assessedwhether the peak response of a given parameteroccurred at one of the intermediate doses andwhether the peak response could be statisticallydistinguished from the response at the highest dose.When both criteria were met, the parameter ofinterest was defined as having a non-monotonicresponse. However, response at the highest dose levelwas still increased compared to controls.Caesarean section on GD 19, males reared by fosterdams, castrated and given testosterone. At 50%increase in free serum estradiol in male mousefoetuses, the prostate was in adulthood enlarged by30% relative to untreated males. As the free serumestradiol concentration in male foetuses wasincreased from 2- to 8-fold, adult prostate weightdecreased relative to males exposed to the 50%increase in estradiol.Dose-related increase in relative uterine weight ingroups 1-4, decreased in group 5-6, but still increasedcompared to group 1. Thus, no anti-oestrogeniceffect. Also, no statistics.Immobility in forced swim test. Decreased at 10 and20 �g/kg and increased at 40 �g/kg.
17-Estradiol
Prostate weight;male/mice
689
1
6-8 litters,1/litter; 5 doses
17-Estradiol
Uterine weight;female/mice
761
2
At least 5 pergroup; 6 doses
17-Estradiol
Antidepressanteffects, measured byimmobility assay;Behaviour/male/mice
762
2
Interference with multiple neuromotortransmitter systems, i.e. dopaminergic andserotonergic
At least 6 pergroup; 5 dosesgiven 45 minbefore testing
41Chemical class17-EstradiolNMDR effect;Organ/sex/speciesNocturnal activity,gene expression inpreoptic area; Brainand behaviour/female/miceRefs.763Group2MoA and other relevant text (mainly asdescribed in the paper)We infer that increases in behaviouralarousal elicited by estrogens are mediatedby changes in the levels of coupledsignaling molecules. Given that treatedanimals have higher motor activity andlower levels of L-PGDS and A2A receptormRNAs in sleep-active areas, thesecorrelational findings support thehypothesis that estradiol may increasebehavioural arousal by decreasing thelevels of well-known sleep-inducingmolecules within the preoptic region.The elevated corticosteronelevels needed to occur in conjunction witha behavioural stress state forcorticosterone-related memoryimpairments to be expressed.??No per group;no. doses12 per group; 7dosesFurther details and remarksRunning wheel activity (RWA) in ovarietectomizedmice increased in groups 2 and 3 and decreased ingroups 4-7 compared to group 3. However, RWA ingroup 7 is increased compared to control. Placed ingroup 2 mainly because ovarietectomized mice wereused.
Corticosterone
Spatial memoryerrors;Behaviour/male/rats
764
2
13 per group; 3and 5 doses
Radial arm water maze. Lower or higher than normallevels of corticosterone caused increased number oferrors.
Corticosterone
Contextual fearconditioning;Behaviour/male/rats
767
2
10 per group; 5doses
Corticosterone
T4
Locomotor activity;Behaviour/male/captive AdeliepenguinsBone growth;Tibia/male/rats withinducedhypothyroidismMemory retention;Behaviour/male/mice
768
3
The results of Experiment 1 indicate thatadministration of corticosterone after memorytraining enhances consolidation of CFC in a dose-dependent manner. The enhancing effect hasessentially an inverted U-function, i.e. onlystatistically significant at dose 4.Adelie penquins - relevance here? Anyway, cannotfind data or figure with NMDR.
771
3
May be due to effect on body weight orgeneral toxicity or a specific effect of T4
10 per group; 5doses
Oxytocin
773
2
Neuromodulator role in theCNS.
10 or 15 pergroup; 6 groups
Made hypothyroid by methimazol. Increase inepiphysial growth at 2, 8 and 32 �g/kg, decrease at64 �g/kg. Similar profile for body weight. Only theright montonic part of the dose-response is likely tobe seen in animals with normal T4 levels1 sc. dose immediately after training, adult mice,receptor antagonist AOT induced a dose-dependentinverted U-shaped increase in retention performance.Relevance for ED uncertain.
42Chemical classDopamineNMDR effect;Organ/sex/speciesMemory;Brain/both/rhesusmonkeyRefs.775Group2MoA and other relevant text (mainly asdescribed in the paper)No per group;no. doses3-5 per group? 4doses for eachsubstanceFurther details and remarksSelective dopamine D1 receptor full agonists A77636and SKF81297 were examined in aged monkeys foreffects on the working memory. Low doses improvedperformance although higher doses impaired or hadno effect on performance. The relevance for ED isuncertain.
PharmaceuticalsDES
Sex ratio, neonatalbody weight, otherneonataldevelopment/both/Mice
777
2
6-10 per group;6 (7) doses
Animals at highest dose could not give birth. %males per litter decreased at the two lowest doses, butsimilar to controls at 3 higher doses. Tendency tomore pups per litter at lowest dose and fewer pupsper litter at highest dose. Birth weight, PD 2 and PD6 weigth decreased at lowest dose and increased at2nd highest or highest dose, however, statistics maynot have considered litter size.Increased prostate weight at dose 3-5, decreased atdose 7. Body weight used as covariate.
DES
Adult prostateweight; Male/mice
689
1
Potential mechanisms mediating adecrease in prostate weight in response tosupraphysiological doses of estrogeninclude receptor down-regulation and thecapacity for estradiol (and possibly otherestrogenic chemicals) to bind to receptorsfor other steroids, such as androgenreceptors, resulting in antagonistic effectsmediated via other receptor systems.
6-8 litters,1/litter; 7 doses
DES
Uterine weight;Female/mice
761
2
At least 5 pergroup; 5 doses
Dose-related increase in relative uterine weight ingroups 1-3, decreased in group 4-5, but still increasedcompared to control. Thus, no anti-oestrogenic effect.Also, no statistics
43Chemical classDESNMDR effect;Organ/sex/speciesMorphologicalparameters;Mammarygland/male andfemale/ miceRefs.779Group2MoA and other relevant text (mainly asdescribed in the paper)Potential mechanisms mediating thereduction in mammary glandgrowth at high doses of DES may includereceptor downregulationand the capacity for oestrogens to bind toreceptors for other hormones – androgenor glucocorticoid receptors,resulting in antagonistic effects mediatedvia other receptor systems in response tosupraphysiological doses of oestrogens.However, the possibility that the effects ofhigh doses of DES are toxic cannot beruled out.The overall dose response of prostate sizeson PNDs 35 and 90 was monotonic; Incontrast to absolute weights, relativeprostate weights (normalized to BW) onPND 35 showed a nonmonotonic doseresponse. Thus, the MoA may be via effecton body weight, e.g. toxicity.No per group;no. doses10-16 immatureper group; 7dosesFurther details and remarksYoung (PD 18) or adults ovarietectomized. Thepercentage area of the mammary fat pad occupied bymammary epithelial structures progressivelyincreased by DES from dose 0.01 �g/day. Themaximum effective dose of DES was 0.1 �g/day bothin young intact and adult OV-X females. However,high dose of DES (10 �g/day had the opposite effect(inverted-U-shaped dose–response curve): mammarysize decreased to control levels. Body weights wererecorded but are not reported. Group 2, becausetoxicity at the high dose cannot be excluded.
Estradiolbenzoate
Dorsal prostateweight, body weight;Male/rats
780
2
SD rats:??F233:6 pergroup and 4doses
On PND 35, there was an increase in prostateweights of SD rats treated with low doses of EB anda decrease in prostate weights of SD rats treated withhigh doses. The low-dose effect was entirelyabolished by PND 90, and only high-dosesuppression of organ sizes was found. The transientnature of the effect in low-dose animals suggests anadvancement of puberty as the cause for increasedreproductive organ weights on PND 35. F344 ratswere more sensitive than SD rats to the suppressiveeffects of high doses of neonatal EB on PND 90.Despite this heightened responsiveness in the F344rats, a low-dose estrogenic effect on adult prostateweights was not observed. Thus, in the rat model asustained effect at low doses of natural estrogens isnot present in the prostate glands.Dose-releated increase in relative uterine weight ingroups 1-5, decreased in group 6-11, but stillincreased compared to control. Thus, no anti-oestrogenic effect. Also, no statistics.
Tamoxifen
Uterine weight;Female/mice
761
2
E
At least 5 pergroup; 11 doses
44Chemical classPlasticsBPANMDR effect;Organ/sex/speciesFertility;Reproductive axis/female/miceRefs.GroupMoA and other relevant text (mainly asdescribed in the paper)No per group;no. doses18-21 dams pergroup; 4 dosesFurther details and remarks
316
2
Continous breeeding. Mainly significant effect at 25�g/kg, i.e. highest dose. No sign of NMDR.However, also some effect on cumulative number ofpups at 25 ng/kg, but not at 50 ng/kg. May be NMDRbut could also be randomNot corrected for litter effects and very few litters.NMDR for some endpoint of male mating behaviour,but not others.Inverse U-shaped curves for vaginal opening,significantly earlier at lowest and highest dose of 0,1and 100 mg/kg, respectively. Those 2 groups had n=5 and 6, respectively.Brain HPOA: Decreased aromatase activity at 0,1and 4 mg/kg, increased at 15, 45, 135 and 405 mg/kgin male offspring. Effect may not be adverse, sogroup 2.PD21-48, The age of preputial separation was41.5 + 0.1 days postpartum in controls (vehicle). The10 mg/kg DEHP dose advanced pubertal onsetsignificantly to 39.7 + 0.1 days postpartum, whereasthe 750 mg/kg DEHP dose delayed pubertal onset to46.3+ 0.1 days postpartum. Similar picture for bw,seminal vesicle weight and serum T.No effects on birth weight. Pup weight PD1 in pupsfor necropsy varies, but N is smaller and litter effectmost likely not considered. Vaginal opening: onlydelayed at the highest doses 15-405 mg/kg. Firstoestrus: no significant effects. Thus no signs ofNMDR.
BPA
Reproductivebehaviours;Behaviour/male/ ratsTiming of vaginalopening, tissueorganization ofuterus; Reproductiveaxis/female/miceAromatase activity;Hypothalamus/male/ratsTiming of puberty;Reproductive axis/male/rats
785
3
2-3 dams (12male offspring)per group; 5dosesControl 48 pergroup, BPA 5-17 per group; 8doses11-12 litters pergroup; 11 doses
BPA
577
2
DEHP
788
2
DEHP
789
1
These data suggest that elevated serumtestosteron levels contributed to precociouspreputial separation in the rats that wereexposed to the low-dose DEHP.
10 per group; 4doses
DEHP
Body weight at birth,vaginal opening, andfirst estrous;Female/rats
790
3
11-16 litters pergroup; 11 doses
45Chemical classDEHPNMDR effect;Organ/sex/speciesRefs.791Group2MoA and other relevant text (mainly asdescribed in the paper)No per group;no. doses4 per group; 4dosesFurther details and remarksPD21-35. Absolute epididymis weight decreased at10 mg/kg, but not at 500 and 750 mg/kg. Absoluteseminal vesicle weight decreased at 10 and 750mg/kg, but not 500 mg/kg. Small group size, i.e. 4per group. Group 2 mainly because of small groupsize.Earlier preputial separation at 40 and 75 mg/kg, laterat 140 mg/kg. DHT serum levels were significantlydecreased at 40 and 75 mg/kg, but not at 140 mg/kg.Group 2 mainly because the effect at highest dosemay be due to general toxicity.
Seminal vesicleweight, epididymalweight, testicularexpression ofsteroidogenesisgenes; Male/ratsDetergents, surfactantsSemicarbazideTiming of preputialseparation, serumDHT; Male/rats
796
2
Unbalance of steroid metabolism,interaction with ER and interference withCNS function at hypothalamic level.Delayed preputial separation at 140 mg/kgmay be related to lower weight, i.e. becaused by general toxicity.Decreased T4 and testosterone may beinvolved.
5 per group; 4doses
UV filtersOctyl methoxy-cinnamate
Activity, memory;Behaviour/both/rats
800
2
11-18 litters pergroup; 4 doses
Activity: increased in males only at 750 mg/kg at 17weeks of age, but not at 9 weeks. Authors state that itmay be a chance finding. Radial arm maze: decreasednumber of errors at 500 and 1000 mg/kg, but not at750 mg/kg.Dose-dependent increase in activity from 600-900mg/kg, some decrease at 1200 mg/kg. Not surprisingas the animals become sedated. Group 3 due totoxicity at highest dose.
Aromatic hydrocarbonsTolueneLocomotor activity;Behaviour/male/rats
801
3
Dopamine-dependent, toluene at thehighest dose (1200 mg/kg) attenuated thespontaneous motor movement andproduced behavioural signs of intoxicationincluding ataxia, which apparentlyinterfered with forward locomotion,thereby resulting in fewer total photocellinterruptions.
5-6 per group; 6doses
46Chemical classPhytoestrogensGenisteinNMDR effect;Organ/sex/speciesAggressive, defensivebehaviours;Behaviour/male/miceRefs.GroupMoA and other relevant text (mainly asdescribed in the paper)Not associated with reduced testes size ordecreased production of testosterone. Maybe combination of mechanismsrelated to sex steroid production andaction. Or related to decreased maternalfood intake and decreased pup weightduring the lactation period, e.g. prenatalprogramming.No per group;no. doses9-14 litters, 1male per litterper group; 3dosesFurther details and remarks
811
1
Decreased body weight during lactation, mainly at 5mg/kg. Not related to litter size as this was smallest at5 mg/kg. Shorter AGD on PD21 at 5 mg/kg, but noeffect on relative AGD. Increased defensivebehaviour at 5 mg/kg, but not at 300 mg/kg. Noeffect on aggressive behaviour, but overall decreasedaggression score, thus indication ofdemasculinization. No effect on mating behaviour,but not an optimal model.Increased memory retention at 30 �g/kg only. Did notincrease the retention latencies of mice that had notreceived a foot shock during training. Relevance forED uncertain.
PhytochemicalsPhlorizin
Memory retention;Behaviour/male/mice
814
2
Competitive inhibitor of glucose transportfrom blood to brain. Acting as a ‘‘glucose-like substance’’ although themechanism(s) of this enhancement isunknown. Inverted U-shape is usual withnumerous other memory-modulatingtreatments.If the observation had been a true hormeticresponse, we would have expected anincrease in litter size at the lower dosesand not a decrease. In rodents, uterinereceptivity to embryos is modulated byovarian estrogen and progesterone. It istempting to propose that some sort ofendocrine modulation is mediating theeffects, however, this proposal isspeculative at this point.
10 or 15 pergroup; 4 doses
HerbicidesCommercialmixture withmecoprop, 2,4-dichlorophenoxyacetic acid anddicamba
Number ofimplantation sites,number of live births;Female/mice
818
2
31-63 litters pergroup fromseveral studies.4 doses
Litter size and implantation sites were significantlyaffected by dose, but resorptions were notsignificantly affected. Implantation sites and littersize in the very low and low doses both differedsignificantly from their control and high doses,respectively. The response varied from season toseason. Group 2 due to uncertainties related toseasonal variation.
47Chemical classSimazineNMDR effect;Organ/sex/speciesEstrous cyclicity;Reproductiveaxis/female/ratRefs.819Group3MoA and other relevant text (mainly asdescribed in the paper)The significant decrease in cycle numbersin the first study with 25 and 100 mg/kgproved not to be significantly differentwith a longer dosing regimen.With suchfew cycles to examine as in study 1, wecannot determine whether the chemical isperturbing long-term cyclicity or if thedelay in onset of VO is causing thetemporary acyclicity, which is commonlyobserved after VO.No per group;no. doses10 per group; 5doses on PD 21-42 and 6 doseson PD 21-62Further details and remarksDelayed vaginal opening from group 3 (monotonic).Estrous cyclicity was generally affected at highestdose. For one of 3 endpoints, i.e. number of cycles,an effect was also found in group 3 (25 mg/kg) in thefirst study. The second study show a similar picture,but the lower number of cycles is not statisticallysignificant. Group 3 as NMDR not seen with longerdosing and thus better data.
InsecticidesDDT
Number of pups, sexratios, neonatal bodyweight, maleanogenital distance;MiceNumber of pups,anogenital distance(males and females),neurobehaviours(males and females);MiceBody weight;Male/rats
777
2
6-10 per group;6 doses
Number of pups per litter, sex ratio and pup bw:some signs of NMDR, but may be due to randomvariation.
Methoxychlor
777
2
Changes in numbers of androgenreceptors? Prostaglandin?
6-10 per group;6 doses
Chlorpyrifos
821
3
9-10 per group;4 doses
Number of pups per litter increased in group 3 only.AGD decreased in both males and females in 2ndhighest dose group, but increased at highest dose.Cliff avoidance latency increased at 2nd highest onPD 2, but not at PD 5. Righting reflex decreased atlowest dose on PD 2, but not PD 5.Male body weight gain appears as showing NMDRwith largest increase in the middle of the dose-response, but there are no statistics related to this andthe effect is quite limited, i.e. max. 108% of controlweight.
48
Appendix 3 – Sensitivity for finding threshold-like doses, based on power analysisThe power of a statistical test is the probability that the test will reject the null hypothesis when thenull hypothesis is false (i.e. the probability of not committing a Type II error, hence the probabilityof not making a false negative decision on whether to reject a null hypothesis). In other words,power is the probability of finding a difference that does exist.The probability of a Type II error occurring is referred to as the false negative rate (β). Thereforepower is equal to 1 − β, which is also known as the sensitivity.Most researchers assess the power of their tests using 0.80 as a standard for adequacy which meansthat the probability for a false negative is less than 0.2. This convention implies a four-to-one trade-off between the probability of a Type II error and a Type I error, when 0.05 is selected as the valuefor statistical significance.Power analysis can be used to calculate the minimum sample size required so that one can bereasonably likely to detect an effect of a given size. Power analysis can also be used to calculate theminimum effect size that is likely to be detected in a study using a given sample size.A number of endpoints relevant for EDs provide quantal data, i.e. they are binary results providinga yes/no answer. Examples of quantal endpoints include malformations of reproductive organs (e.g.hypospadias), fertility index, pregnancy index etc. Results of histopathological evaluation may bereported either as yes/no answers or as distribution among scores from e.g. 0-3. Nipple retention is ayes/no endpoint if it is expressed as the number of males with or without nipple, but this endpointcan also be semi-quantitative, if the number of nipples is recorded (i.e. from 0 to 12).Assessment of quantal endpoints is generally expected to lead to a lower power than assessment ofcontinuous endpoint. To explore this, we have calculated the effect size needed for findingsignificant effect, i.e. p < 0.05, for yes/no endpoints (Table 2) and continuous endpoints (Table 3).This was done for studies with 8 litters per group, because that is the group sizes expected in theOECD TG 421/422 Reproductive Toxicity Screening study which is used for REACH testing at thetonnage level of 10 tpa. In addition it was done with 20 litters per group as this is the expectednumber per group in the OECD TG 416 and OECD TG 443. As the evaluation of some endpointsmay be done in more than one offspring per litter, the calculations for the quantal endpoints alsoillustrate the effect sizes needed when 2 or 5 offspring per litter is assessed. However, the correcteffects sizes needed for 2 or 5 animals per litter are likely to be higher than the ones shown as ourcalculation is based on the offspring as the statistical unit. To be correct, the calculations should bebased on the litter as the statistical unit, i.e. the method should have corrected for litter effects. Thiswas unfortunately not possible for us as there to our knowledge are no available easily usedstatistical programs for that purpose for quantal data.
49
Table 2. Effect sizes for quantal endpoints needed for p value < 0.05 in one-tailed Fisher Exact test*
Increase of effect size
Pups
No.
compared to historical
per
control of 0.3% for
Litters
No. with without
effect
per group litter
effect
Group
Effect size hypospadias
201 Control0200%
201 Exposed5158333%25%
202 Control0400%
202 Exposed5354167%13%
205 Control01000%
205 Exposed5951667%5%
81 Control080%
81 Exposed4416667%50%
82 Control0160%
82 Exposed51110417%31%
85 Control0400%
85 Exposed5354167%13%
*The statistics used when more than one male pup per litter is included is based on using the pup as thestatistical unit. Generally, the litter is considered as the correct statistical unit in developmental toxicitystudies and using this approach will in most cases lead to even higher effect sizes than those shown in thetable.Quantal dataThe results in table 2 show that for having a statistically significant effect with 20 litters per groupthe frequency of effect in the exposed group has to be 25% with 1 male per litter, 13% with 2 malesper litter and 5% with 5 males per litter. With 8 litters per group the frequency of effect in theexposed group has to be 50% with 1 male per litter, 31% with 2 males per litter and 13% with 5males per litter.The frequency of hypospadias in humans is around 1 of 300, i.e. 0.3% (Moore 1983). Based on ourhistorical control values for male external genital malformations, incl. hypospadias in Wistar rats,we actually find a similar frequency of 0.32% in rats (1 of 308). The effect sizes needed for findinga significant effect compared to this historical control frequency showed that the increase of thefrequency has to be very large, i.e. 17-170 fold depending on the number of litters per group and thenumber of males studies per litter (Table 2).We have in our calculations assumed that there were no offspring with hypospadias observed in thecontrol group and this will with a historical control value for hypospadias around 0.3% be themajority of cases. However, in some few cases one hypospadias may occur in the control group andthe effect sizes needed for finding a significant effect will become around 50% higher (data notshown) and the increase compared to the historical control value for hypospadias will be similarlyincreased to around 25-250 fold (data not shown). This very low sensitivity for detecting significanteffects on rare adverse outcomes is generally recognized for malformations. Thus, the occurrence of
50
a few similar rare malformations such as hypospadias may generally be considered toxicologicallyrelevant although the finding is not statistically significant.For other quantal endpoints such as histopathology, fertility index, pregnancy index etc. one or afew cases may occur in the control group and there will often only be one data point per litter. Thusfor these endpoints, the effect size needed for finding a significant effect may often be around 50%higher than those in table 3, i.e. range from 25-37% with 20 animals per group and from 50-75%with 8 animals per group. This very low sensitivity is often not considered when evaluating suchendpoints.Overall, the results illustrate that the effect size for a quantal effect has to be high in studies with 20litters per group i.e. 25-37% and even higher with only 8 litters per group, i.e. 50-75%. This impliesthat the sensitivity for detecting quantal effects is very low and that effects sizes of human relevancemay be present at the NOAEL.Continuous effect dataThe statistical power for detecting significant effects on continuous endpoints depends on the groupsize and the coefficient of variation (CV) in the control group. Generally, 80% or higher power isregarded as sufficient. The CV is a normalized measure of dispersion of a probability distribution.It is also known as the variation coefficient. The CV is also sometimes known as relative standarddeviation (RSD), which is expressed as a percentage, i.e. it is calculated as the sample standarddeviation divided by the sample mean and multiplied by 100. The results of power calculationsbased on different groups sizes and CVs using the shareware program C*3.1.3 are shown in table 3.For AGD, the CV is normally around 5- 7% and an effects size of ca. 4-7% will in most cases bestatistically significant in studies with N=20 per group, whereas the effect size has to be 7-11% ifonly 8 litters per group are studied. For endpoints with higher CV’s such as 10% or 12%, the effectssizes also have to be higher, i.e. 9-11% for 20 litters per group and 15-18% for 8 litters per group.These results illustrate that the effect sizes needed for detecting effects with N=8 is approximately1.6-1.8 times higher than when N=20.Continuous effect data are generally expected to be more sensitive than quantal data and this is alsofound here as the effect sizes needed for continuous data range from 4-18%, whereas the effectsizes for quantal data in the previous section was found to range from 25-75%.Table 3. Effect sizes for continuous data needed for power > 79% and with p<0.05CV
N=20
N=8
Ratio
4.5%4%7%1.87.1%7%11%1.610.0%9%15%1.712.0%11%18%1.6CV = Coefficient of variation (standard deviation/group mean*100 for control group)