Sundheds- og Forebyggelsesudvalget 2011-12
L 161
Offentligt
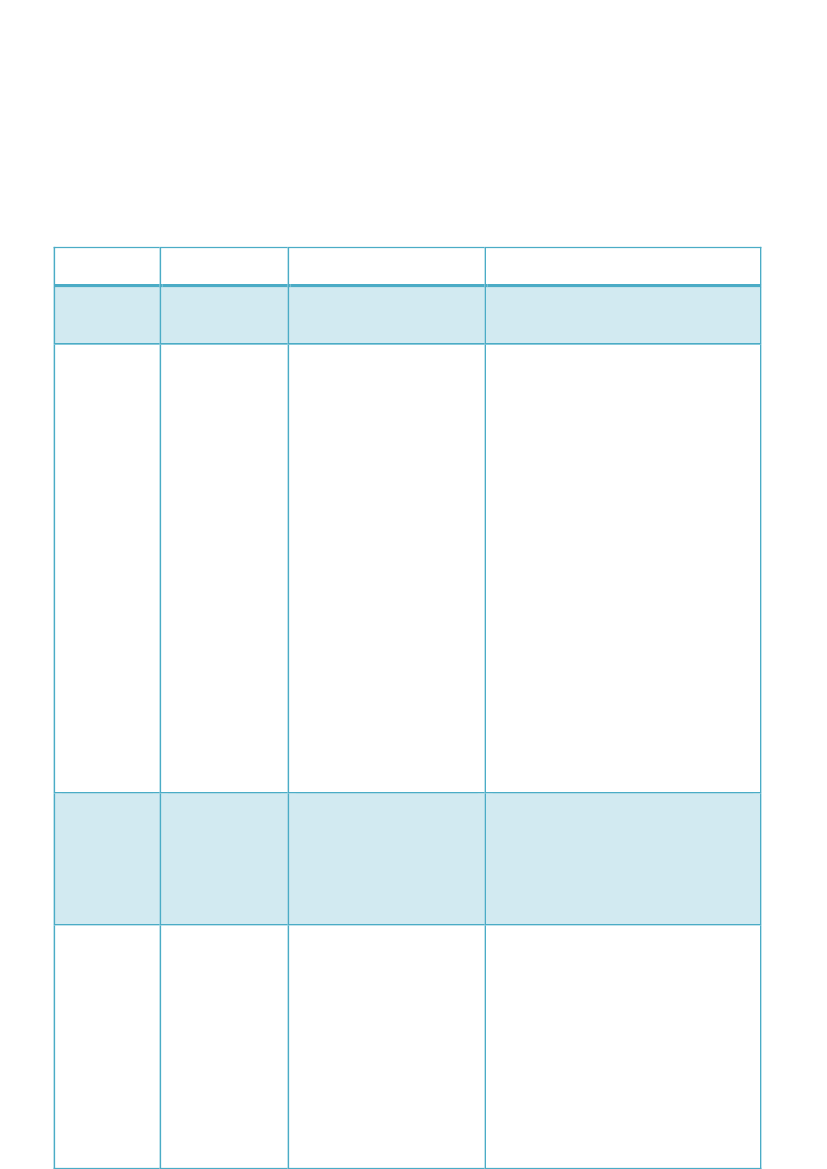


Answers to questions on consequences of EMA's postponement of certainPhV activities on national implementation1. Do you postpone the implementation of certain provisions in the new PhV legislation dueto EMA’s postponements?2. If yes, please describe which provision(s)?3. If no, why not? And how would you enforce provisions where the underlying proceduresare not functioning?CountryFinlandPostponing, yesor no?NoIf Yes, please describewhich provision(s)If No, why not?The Finnish national legislation does notdetail the European procedures describedin articles 107 e, 107 g and 107 n – 107 q.The French national legislation does notdetail the Europeans procedures ofevaluation (procedures described inArticles 107e and 107g regarding thesingle assessment of PSURs and the partsof Articles 107n to 107q regarding theparticipation of PRAC); it is regarded asinstitutional obligations whichimposedde factoto our Agency.Indeed, for example, only the obligationsto submit to the EMA PSURs and theircontent are implemented.Moreover, according to the article 2 (7) ofthe Directive 2010/84/EU, our lawprovides that these new provisions shallenter into force from 12 month after thefunctionalities of the repository havebeen established and have beenannounced by the EMA. Until the EMAcan ensure the functionalities agreed forthe repository of the PSURs, the MAHshall submit the PSURs to the FrenchAgency.Because the Member States are legallyobliged to implement all provisions intime. In case the underlying Europeanprocedures are not functioning, we (PEI)may do the work ourselves (cannot tellyou in detail at the moment).We will postpone all theactivities that EMA ispostponing (i.e. proceduresdescribed in Articles 107eand 107g regarding thesingle assessment of PSURsand parts of Articles 107n to107q regardingthe participation of PRAC inthe non-interventional PASSassessment). EMA's activeparticipation is considerednecessary for
France
No
Germany
No (as far as JanFarzan knows)
Greece
Yes, we intend topostpone theactivities/procedures in co-ordination withEMA
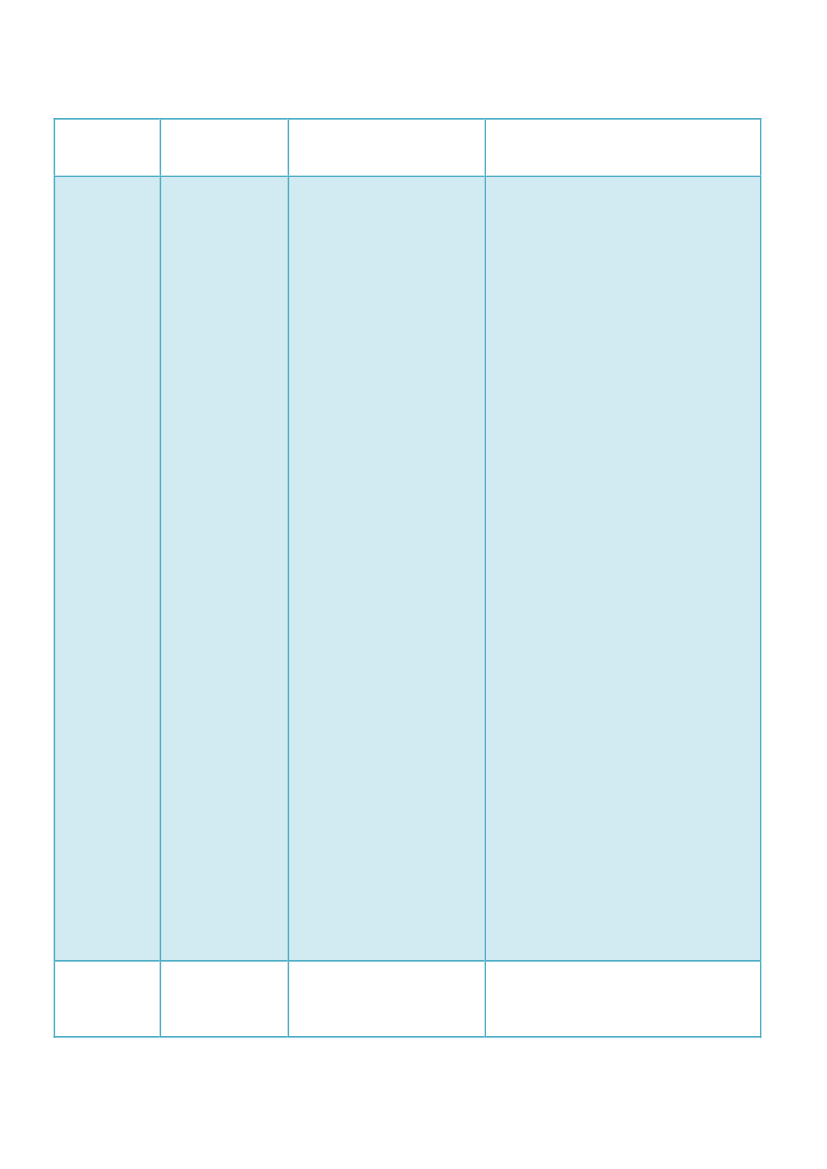
the implementation of theabove-mentionedprocedures.HungaryNoNo, Hungary will not postpone theimplementation of the provisions of theDirective due to postponements of EMA.As a Member State, we are legally obligedto implement all provisions in time.Postponing implementation of theDirective by Hungary could be regardedas an infringement of the Europeanlegislation.However active participation of EMA isconsidered necessary for theimplementation of mentionedprocedures, where the provisions areworked out, we will execute and enforcerelevant provisions according to relevantnational legislation.Implementation in Hungarian legislationcovers obligations for MAH, not theprocedures of PRAC or the co-ordinationgroup. If there is a cross-reference to thePRAC or to the coordination group,obligations of the MAH will be supervisedand controlled by the National Instituteof Pharmacy of National Institute forQuality- and Organizational Developmentin Healthcare and Medicines (GYEMSZIOGYI) as the competent authority withinits scope of authority, according to therelevant national legislation in force, untilEMA can ensure the functionalitiesagreed for the repository of the PSURs.For practical purposes - until EMAannounces the functionality of itsrepository is established - we will receivePSURs directly.Our intent only was to transpose thoseaspects those directly relate to theobligations of the MAH and thecompetent authority, towards to makeclear to MAH its tasks with respect tosubmission of PSURs, draft protocols,protocol amendments and etc.AIFA doesn’t believe it is legally possiblenot to implement the proceduresdescribed in Articles 107e and 107gregarding the single assessment of PSURs
Italy
No
LatviaLiechtenstein
NoNo(?)
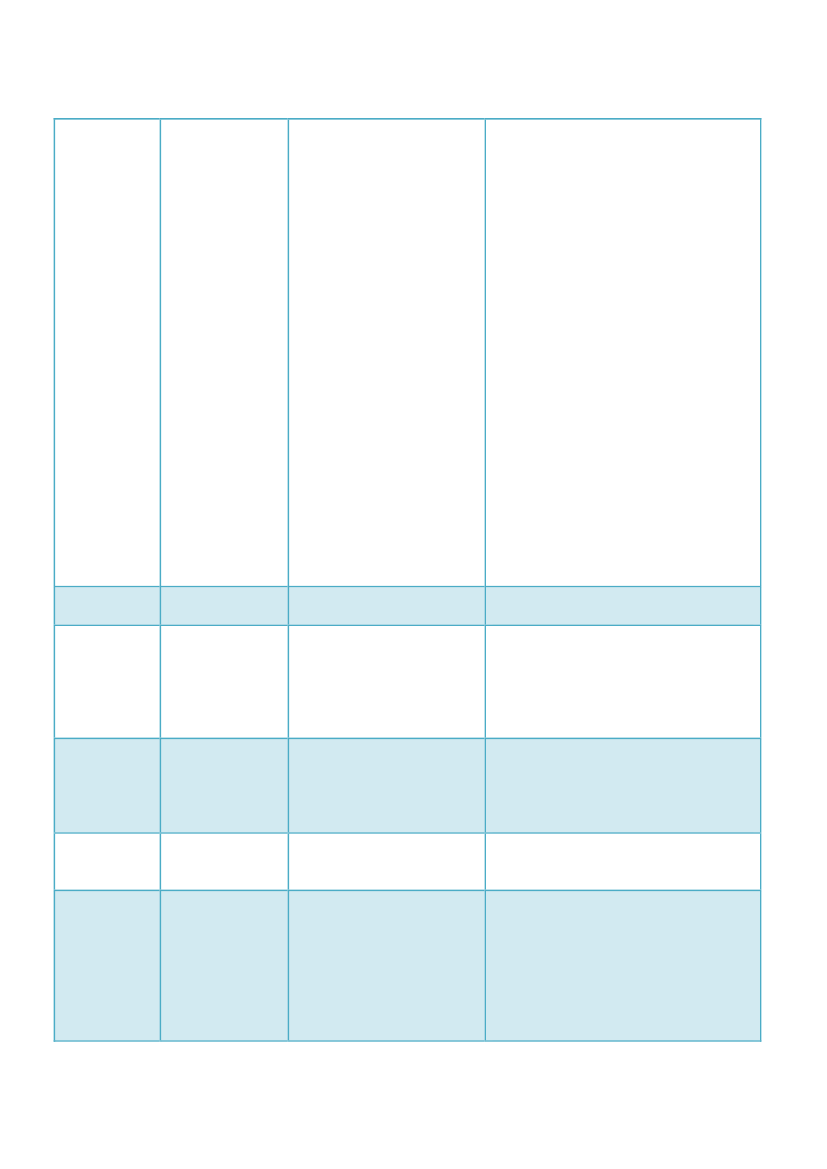
Netherlands
No(?)
Spain
No
Sweden
No
and the parts of Articles 107n to 107qregarding the participation of PRAC(endorsement of protocols, amendmentsthereof and results management forNAPs) taking into account the EMA’spostponement.In the last meeting held in December, theMB decided the deadline of theimplementation of the provisions of theDirective 2010/84/EU. The decision waspublished on February the 2�.Apart from EMA’s decision to postponecertain PhV activities due to the lack offund, we are aware to be obliged toadopt PhV activities since the entry inforce of the Directive 2010/84/EU.Directives, in fact, are binding for theMember States to which they refer.Member States shall adopt all theactivities for their implementation in theform and at the time indicated.By the way, the postponement of certainactivities by the MS, could be regarded asan infringement of the Europeanlegislation.Our legislation will transpose theDirective.The new pharmacovigilance regulationand directive are still in the process ofbeing included into the EEA legislation.As they are still not part of the EEAacquis, the question of postponementdoes not arise at the moment.The conversion from directive intonational law is undiminished in progressand will be round up accordingly. In duecourse, when the provisions are workedout, they will be executed and enforced.Our legislation will transpose theDirective. We will issue a document withtransitional arrangements.Sweden will not postpone theimplementation of the provisions of thedirective due to EMAs postponements.The implementation in Swedishlegislation covers obligations for MAHand the MPA, not the procedures of PRACor the co-ordination group.
UnitedKingdom
No, we do notintend topostponeimplementationof the provisionsrelating to thePSUR singleassessment or theprovisionsrelating to thepost-authorisationsafety studies.
Our approach to transposition intonational legislation has been to onlytranspose those aspects that directlyrelate to the obligations of the MAH andthe competent authority.Although the single PSUR assessment willnot fully come in to force in July 2012, thecurrent worksharing scheme will stillcontinue for those products alreadysubject to this scheme and for all othernational products these PSURs will beassessed at a national level. We thereforeenvisage that the approach toassessment of PSURS for nationallyauthorised products will in the interimremain unchanged from what currentlyhappens. The transitional measures willmean that we will receive PSURs directlyand this will continue until 12 monthsafter the EMA has announced thefunctionality of its repository have beenestablished. We do believe that thispostpone of implementation will causeany major difficulties but we will need tomake clear to MAHs our expectationswith respect to submission of PSURs.The new procedures for submission andassessment of PASS protocol, substantialamendments and final study results asprovided for in Articles 107n to 107q ofDirective 2011/83/EC only apply to PASSstudies which have been imposed after21 July 2012 as a condition of themarketing authorisation. Therefore wedo not believe that there will be largenumbers of these studies and if it is notpossible for them to be considered byPRAC we would imagine that these wouldbe handled through a written procedurewith the Reference Member State takingthe responsibility for the assessment. Themost recent Q&A documents on theimplementation of the pharmacovigilancelegislation issued by the EMA (dated 23rdMay 2012) do not provide clarity onwhether they expect study protocols tobe submitted to them. Nevertheless it
will be important that we provide clearinformation to MAHs about what ourexpectation are with respect tosubmission of draft protocols, protocolsamendments and final study reports.





