Sundheds- og Forebyggelsesudvalget 2011-12
SUU Alm.del Bilag 172
Offentligt
DCCT and EDIC:
National Diabetes Information Clearinghouse
The Diabetes Control and ComplicationsTrial and Follow-up StudyWhat is the DCCT?The Diabetes Control and ComplicationsTrial (DCCT) was a major clinical study con·ducted from 1983 to 1993 and funded by theNational Institute of Diabetes and Digestiveand Kidney Diseases. The study showedthat keeping blood glucose levels as closeto normal as possible slows the onset and pro·gression of the eye, kidney, and nerve damagecaused by diabetes. In fact, it demonstratedthat any sustained lowering of blood glucose,also called blood sugar, helps, even if the per·son has a history of poor control.The DCCT involved 1,441 volunteers, ages13 to 39, with type 1 diabetes and 29 medi·cal centers in the United States and Canada.Volunteers had to have had diabetes for atleast 1 year but no longer than 15 years. Theyalso were required to have no, or only earlysigns of, diabetic eye disease.The study compared the effects of standardcontrol of blood glucose versus intensivecontrol on the complications of diabetes.Intensive control meant keeping hemoglobinA1C levels as close as possible to the normalvalue of 6 percent or less. The A1C blood testreflects a person’s average blood glucose overthe last 2 to 3 months. Volunteers were ran·domly assigned to each treatment group.
What is the EDIC?When the DCCT ended in 1993, research·ers continued to study more than 90 percentof participants. The follow-up study, calledEpidemiology of Diabetes Interventions andComplications (EDIC), is assessing the inci·dence and predictors of cardiovascular diseaseevents such as heart attack, stroke, or neededheart surgery, as well as diabetic complica·tions related to the eye, kidney, and nerves.The EDIC study is also examining the impactof intensive control versus standard control onquality of life. Another objective is to look atthe cost-effectiveness of intensive control.
U.S. Departmentof Health andHuman ServicesNATIONALINSTITUTESOF HEALTH
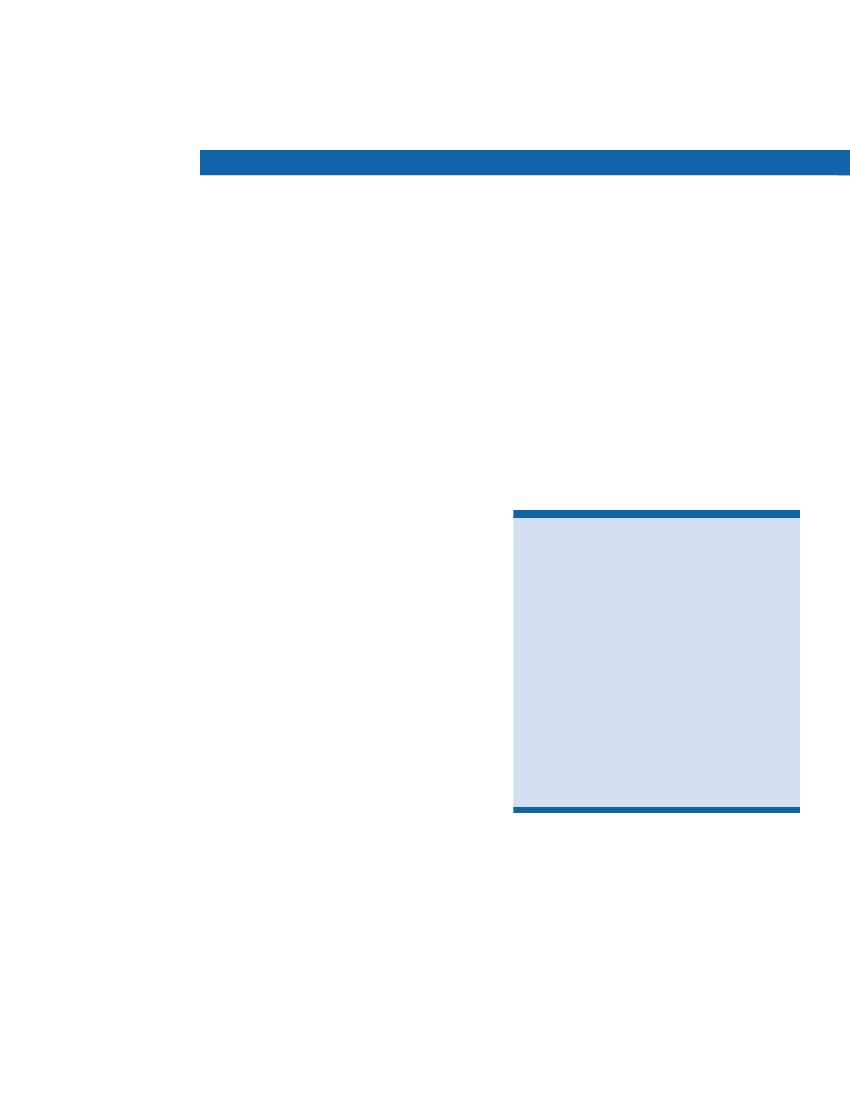
DCCT Study FindingsIntensive blood glucose control reduces
risk of
• eye disease 76% reduced risk• kidney disease 50% reduced risk• nerve disease 60% reduced risk
EDIC Study FindingsIntensive blood glucose control reduces
risk of
• any cardiovascular disease event 42% reduced risk• nonfatal heart attack, stroke, or death from cardiovascular causes57% reduced risk
How did intensive treatmentaffect diabetic eye disease?All DCCT participants were monitored fordiabetic retinopathy, an eye disease thataffects the retina. Study results showedthat intensive therapy reduced the riskfor developing retinopathy by 76 percent.In participants who had some eye damageat the beginning of the study, intensivemanagement slowed the progression ofthe disease by 54 percent.The retina is the light-sensing tissue at the backof the eye. According to the National Eye Insti·tute, one of the National Institutes of Health, asmany as 24,000 people with diabetes lose theirsight each year. In the United States, diabeticretinopathy is the leading cause of blindness inadults less than 65 years of age.
How did intensive treatmentaffect diabetic nerve disease?Participants in the DCCT were examined todetect the development of nerve damage, ordiabetic neuropathy. Study results showedthe risk of nerve damage was reduced by60 percent in people on intensive treatment.Diabetic nerve disease can cause pain and lossof feeling in the feet, legs, and fingertips. Itcan also affect the parts of the nervous systemthat control blood pressure, heart rate, diges·tion, and sexual function. Neuropathy is amajor contributing factor in foot and legamputations among people with diabetes.
How did intensive treatmentaffect diabetic kidney disease?Participants in the DCCT were tested toassess the development of diabetic kidney dis·ease, or nephropathy. Findings showed thatintensive treatment prevented the develop·ment and slowed the progression of diabetickidney disease by 50 percent.Diabetic kidney disease is the most commoncause of kidney failure in the United States.After having diabetes for 15 years, one-thirdof people with type 1 diabetes develop kidneydisease. Diabetes damages the small bloodvessels in the kidneys, impairing their abilityto filter impurities from blood for excretionin the urine. People with kidney failure musthave a kidney transplant or rely on dialysis tocleanse their blood.
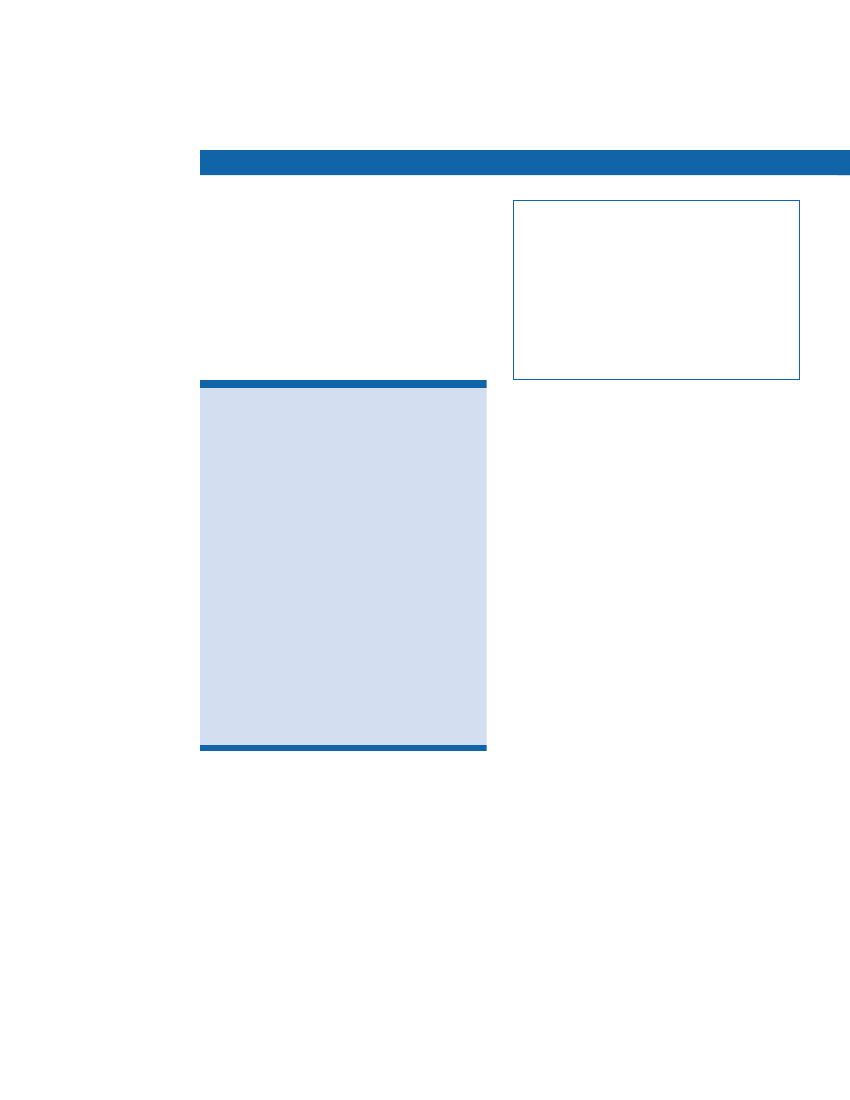
Elements of IntensiveManagement in the DCCT• Testing blood glucose levels four or more times a day• Injecting insulin at least three times daily or using an insulin pump• Adjusting insulin doses according to food intake and exercise• Following a diet and exercise plan• Making monthly visits to a health care team composed of a physician, nurseeducator, dietitian, and behavioraltherapist
2
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study
How did intensive treatmentaffect diabetes-relatedcardiovascular disease?People with type 1 diabetes have a tenfoldgreater risk of heart disease compared withnondiabetic patients because high bloodglucose can damage the heart and bloodvessels. That damage can lead to heartattacks and strokes, the leading causes ofdeath for people with diabetes.Another condition related to heart diseaseand common in people with diabetes isperipheral arterial disease (PAD), also calledperipheral vascular disease (PVD). Withthis condition, the blood vessels in the legsare narrowed or blocked by fatty deposits,decreasing blood flow to the legs and feet.PAD is a sign of widespread atherosclerosis,and people with PAD are at increased riskof heart attack or stroke. Poor circulationin the legs and feet also raises the risk ofamputation.When the initial findings of the DCCT wereannounced in 1993, it was too early to detectthe effects of the therapies on cardiovasculardisease because patients were young. In2005, however, EDIC researchers reportedthat the risk of any heart disease was reducedby 42 percent in people who had been in theintensive treatment group. Volunteers inthe intensive treatment group also cut theirrisk of nonfatal heart attack, stroke, or deathfrom cardiovascular causes by 57 percent.
Patients received the intensive therapy foran average of 6.5 years in the DCCT. Morethan 10 years after the DCCT ended, whenboth groups began receiving similar care, thebenefits to the heart of the earlier treatmentemerged. Moreover, the EDIC study foundthe benefits of tight glucose control on eye,kidney, and nerve problems persisted longafter the DCCT ended. Researchers call thelong-lasting benefit of tight control “meta·bolic memory.” Following the DCCT, bloodglucose levels in the intensive treatment grouprose, and those of the conventional treatmentgroup declined, so that blood glucose levelsare now nearly the same between treatmentgroups.
What are the risks of intensivetreatment?In the DCCT, the most significant side effectof intensive treatment was an increase in therisk for hypoglycemia, also called low bloodglucose, including episodes severe enough torequire assistance from another person.When blood glucose falls too low, a personcan become confused, behave irrationally,have seizures, lose consciousness, or even die.The good news is that such episodes, whiledangerous at the time, do not lead to a long-term loss of cognitive function—the ability toperceive, reason, and remember—as scien·tists originally feared. Researchers recentlyreported this finding after examining 1,144of the original DCCT participants a mean of18 years after enrollment in the DCCT.
3
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study
The DCCT did not study intensive therapy inyoung children or in patients with severe com·plications, frequent hypoglycemia, or thosewith a limited life expectancy. While mostpatients benefit from keeping their bloodglucose levels as close to normal as possible,less stringent goals may be appropriate forsome patients.DCCT researchers estimate that intensivemanagement doubles the cost of managingdiabetes because of increased visits to a healthcare professional and the need for more fre·quent blood testing at home. However, thiscost is offset by the reduction in medicalexpenses related to long-term complicationsand by the improved quality of life of peoplewith diabetes.
What do the results of the DCCTand EDIC studies mean forpeople with type 2 diabetes?Results of the DCCT and EDIC studieshave important implications for preventingdiabetes complications in people withtype 2 diabetes because the microvasculardisease development process is likely to besimilar for both type 1 and type 2 diabetes.One study of people with type 2 diabetes, theUnited Kingdom Prospective Diabetes Study,demonstrated that controlling blood glucoselevels reduced the risk of diabetic eye diseaseand kidney disease.Other studies of the role of blood glucosecontrol in people with type 2 diabetes arestill under way. For example, the Action toControl Cardiovascular Risk in Diabetes
(ACCORD) trial, a multicenter, randomizedtrial, is studying approaches to preventingmajor cardiovascular events in individualswith type 2 diabetes. ACCORD is designedto compare current practice guidelines withmore intensive glycemic control in 10,000individuals with type 2 diabetes, includingthose at especially high risk for cardiovasculardisease (CVD) events because of age,evidence of subclinical atherosclerosis, orexisting clinical CVD. More intensive controlof blood pressure than is called for in currentguidelines and a medication to reduce triglyc·eride levels and raise HDL cholesterol levelswill also be studied in subgroups of these10,000 volunteers. Each treatment strategywill be accompanied by standard adviceregarding lifestyle choices, including diet,physical activity, and smoking cessation,appropriate for individuals with diabetes.The primary outcome to be measured isthe first occurrence of a major CVD event,specifically heart attack, stroke, or cardio·vascular death. In addition, the study willinvestigate the impact of the treatment strate·gies on other cardiovascular outcomes; totalmortality; limb amputation; eye, kidney, ornerve disease; health-related quality of life;and cost-effectiveness.In February 2008, the National Heart, Lung,and Blood Institute decided to stop one partof the study—the intensive glycemic controltreatment—before the end of the entire trialbecause of safety concerns. However, the trialwill continue with the other treatments untilthe planned end in 2009.
4
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study
What are the most importantfactors in preventing diabetescomplications?Research studies have shown that control ofblood glucose, blood pressure, and blood lipidlevels helps prevent complications in peoplewith type 1 or type 2 diabetes.
You may also find additional information about thistopic visiting MedlinePlus atwww.medlineplus.gov.This publication may contain information aboutmedications used to treat a health condition. Whenthis publication was prepared, the NIDDK includedthe most current information available. Occasion·ally, new information about medication is released.For updates or for questions about any medications,please contact the U.S. Food and Drug Administra·tion at 1–888–INFO–FDA (463–6332), a toll-free call,or visit their website atwww.fda.gov.Consult yourdoctor for more information.
Results of the DCCT are reported in theNew England Journal of Medicine,329(14),September 30, 1993.Results of the EDIC are reported intheNew England Journal of Medicine,353(25), December 22, 2005.Reprints of articles related to the DCCTor the EDIC can be ordered from theNational Diabetes Information
Clearinghouse
1 Information WayBethesda, MD 20892–3560Phone: 1–800–860–8747Fax: 703–738–4929Email: [email protected]Internet: www.diabetes.niddk.nih.gov/statistics/reprints.htm
5
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study
National DiabetesInformation Clearinghouse1 Information WayBethesda, MD 20892–3560Phone: 1–800–860–8747Fax: 703–738–4929Email: [email protected]Internet: www.diabetes.niddk.nih.govThe National Diabetes Information Clearinghouse(NDIC) is a service of the National Institute of Dia·betes and Digestive and Kidney Diseases (NIDDK).The NIDDK is part of the National Institutes ofHealth of the U.S. Department of Health and HumanServices. Established in 1978, the Clearinghouseprovides information about diabetes to people withdiabetes and to their families, health care profession·als, and the public. The NDIC answers inquiries,develops and distributes publications, and worksclosely with professional and patient organizationsand Government agencies to coordinate resourcesabout diabetes.Publications produced by the Clearinghouse are care·fully reviewed by both NIDDK scientists and outsideexperts.
This publication is not copyrighted. The Clearing·house encourages users of this fact sheet to duplicateand distribute as many copies as desired.This fact sheet is also available atwww.diabetes.niddk.nih.gov.
The U.S. Government does not endorse or favor anyspecific commercial product or company. Trade,proprietary, or company names appearing in thisdocument are used only because they are considerednecessary in the context of the information provided.If a product is not mentioned, the omission does notmean or imply that the product is unsatisfactory.
U.S. DEPARTMENT OF HEALTHAND HUMAN SERVICESNational Institutes of Health
NIH Publication No. 08–3874May 2008