Miljø- og Planlægningsudvalget 2010-11 (1. samling)
B 41
Offentligt
POLICY ANALYSISpubs.acs.org/est
120 Years of Nanosilver History: Implications for Policy MakersBernd Nowack,*,†Harald F. Krug,†and Murray Height‡†‡
EMPA-Swiss Federal Laboratories for Materials Science and Technology, Lerchenfeldstrasse 5, CH-9014 St. Gallen, SwitzerlandHeiQ Materials AG, CH-5330 Bad Zurzach, Switzerland
SbSupporting Information
ABSTRACT:Nanosilver is one nanomaterial that is currently under a lot of scrutiny. Much of the discussion is based on theassumption that nanosilver is something new that has not been seen until recently and that the advances in nanotechnology openedcompletely new application areas for silver. However, we show in this analysis that nanosilver in the form of colloidal silver has beenused for more than 100 years and has been registered as a biocidal material in the United States since 1954. Fifty-three percent of theEPA-registered biocidal silver products likely contain nanosilver. Most of these nanosilver applications are silver-impregnated waterfilters,algicides, and antimicrobial additives that do not claim to contain nanoparticles. Many human health standards for silver arebased on an analysis of argyria occurrence (discoloration of the skin, a cosmetic condition) from the 1930s and include studies thatconsidered nanosilver materials. The environmental standards on the other hand are based on ionic silver and may need to be re-evaluated based on recentfindingsthat most silver in the environment, regardless of the original silver form, is present in the form ofsmall clusters or nanoparticles. The implications of this analysis for policy of nanosilver is that it would be a mistake for regulators toignore the accumulated knowledge of our scientific and regulatory heritage in a bid to declare nanosilver materials as new chemicals,with unknown properties and automatically harmful simply on the basis of a change in nomenclature to the term“nano”.
’INTRODUCTIONThe potential adverse effects of nanoparticles on humans andthe environment currently receive a lot of attention both inacademia and with regulators.1,2A lot of the discussion is centeredon the asserted assumption that nanoparticles are somethingfundamentally“new”and thus cannot be compared to conventionalchemicals or bulk materials. Nanosilver is one of the nanomaterialsthat is under the most scrutiny today3-5and its release and effectsare studied widely.6-9Although changes in nomenclature over thedecades have created confusion among scientists and policymakers, it is undeniable that products containing nanoscale silverparticles have been commercially available for over 100 years andwere used in applications as diverse as pigments, photographics,wound treatment, conductive/antistatic composites, catalysts,and as a biocide. With this long and diverse history of use it isclear that an extraordinary amount of research into the chemistryof nanoscale silver has been conducted over the past 120 years;it should be noted that most research, until very recently, did notuse“nano”nomenclature.In this analysis we critically examine with respect to nanosilverthree important assertions often made when discussing riskassessment of silver nanoparticles:(1) Nanosilver is new and exhibits unique physical andchemical properties compared to“conventional”silver(e.g., macroscale“bulk”silver).(2) Nanosilver has been used for only a few years and theenvironment and humans have never been exposed tonanosilver before.(3) Existing risk assessments of silver have been based on adata set derived from conventional silver materials, sothey do not apply to nanosilver.r2011 American Chemical Society
’ANTIMICROBIAL BIOCIDESAntimicrobial biocides are commonly used to prevent thegrowth of bacteria on surfaces and within materials and aretypically added in small quantities to many applications to makeit more difficult for bacteria to grow on the treated object. Bio-cidal functionality can be achieved by employing either organicor inorganic active agents. Compounds such as quaternaryammonium and chlorinated phenols are examples of two widelyused organic chemical biocidal agents. Inorganic active agents aregenerally based on metals such as silver and copper. Silver hasfound a growing presence in many applications due to a desire toshift away from organic chemical agents toward additives, whichcan be used in much lower concentrations in a wider variety ofproducts including applications such as plastics where high-temperature processing is not feasible for organic compounds.Examples of applications are bacteriostatic waterfiltersforhousehold use10or swimming pool algicides.11To meet thediversity of application types, many different forms of silvercompounds have been developed to service this market.Whereas biocidal action derives from interaction of silverions with bacteria, silver additives are differentiated primarily bythe way the silver ions are stored in the product. Common silverproducts range from additives that store and release discretesilver ions held within a ceramic (e.g., zeolite) or glass matrix,through to products that store silver ions as silver salts (e.g.,silver chloride) or elemental silver (e.g., nanoscale silver metal).Received:September 30, 2010Accepted:December 16, 2010Revised:December 13, 2010Published:January 10, 20111177dx.doi.org/10.1021/es103316q|Environ. Sci. Technol.2011, 45, 1177–1183
Environmental Science & Technology
POLICY ANALYSIS
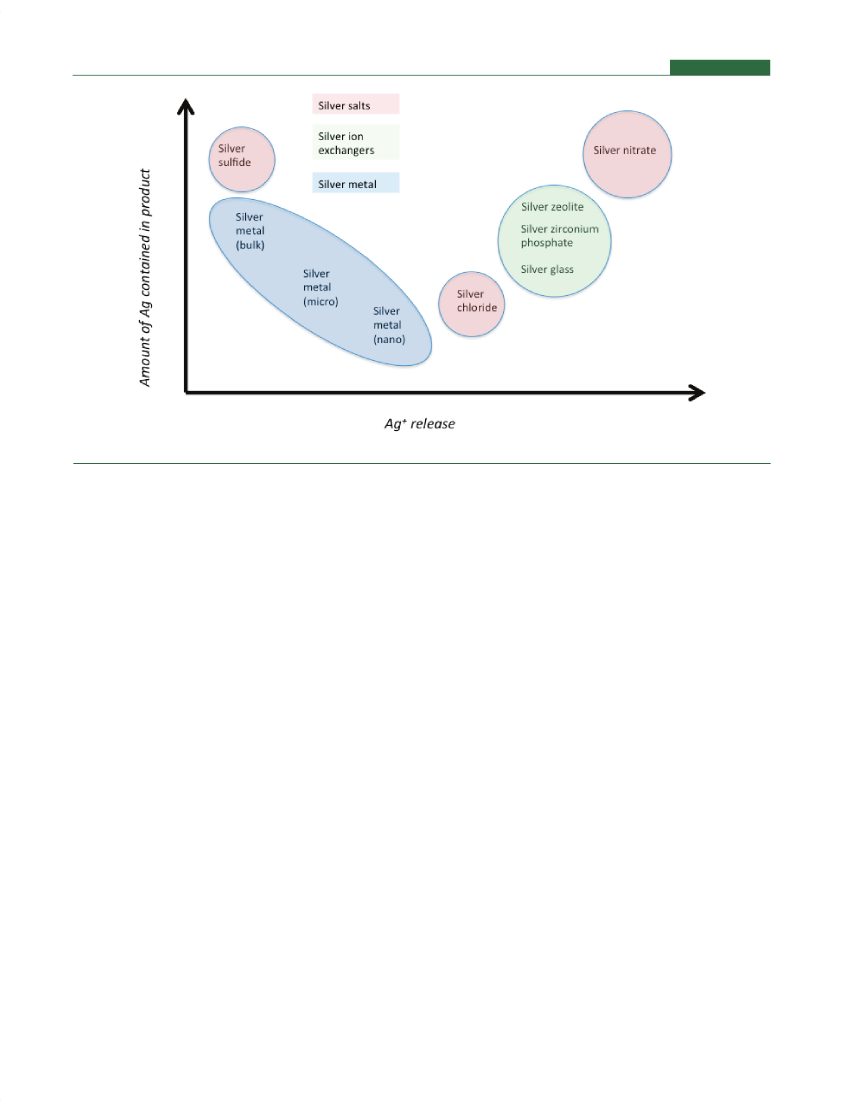
Figure 1.Silver release and amount of silver required in products for different biocidal silver formulations.
Upon contact with moisture, silver ions are released from theadditive and the object treated with the additive. The biocidalpotency of a silver additive is therefore directly related to thepotential for releasing silver ions.12,13However, in the case offree nanosilver particles the interactions can potentially bemore complex, and catalytic reactions on the particle surfacethat can change as a function of size and shape of the nano-particles can complicate the system.14It should be noted,however, that most commercial applications of nanosilverinvolve embedding the particles within a matrix such as a plasticor a coating.There are two clear“bookends”for illustrating the extremes ofthe potential for silver ion release from silver substances: namelysilver sulfide (highly insoluble, hence a low potential for silver ionrelease) and silver nitrate (completely soluble, maximum poten-tial for silver ion release). The release potential of different silvermaterials can be distributed between the silver sulfide and silvernitrate extremes (Figure 1). Materials that store discrete silverions in a matrix show a high potential for releasing silver ions,only marginally less than silver nitrate. Silver salts such as silverchloride show a lower release potential than the ion-basedmaterials and so are positioned further from silver nitrate. Atthe other extreme, bulk silver metal (e.g., silver ingot) releasessilver ions to a small extent and so has a potential closer to thesilver sulfide extreme. As the size of silver metal is decreased frombulk through to micrometer-sized particles through to nanosizedparticles, the potential for releasing silver ions increases becauseof increasing surface availability per mass of silver and becauseboth the solubility and dissolution kinetics of silver may vary as afunction of size as silver metal size decreases. Therefore thepotential for releasing silver ions increases and so the behaviormoves away from the silver sulfide bookend toward silver nitrate.It is important to note that while the tendency for higher silverion release improves with smaller silver particle size, the silversalts and silver-ion materials still show higher potential andantimicrobial activity than the nanosized silver metal materials.12
’HISTORY OF NANOSILVER PRODUCTION AND USEOne of the standard definitions of nanotechnology encom-passes“researchand technology development at the atomic,molecular, or macromolecular levels using a length scale ofapproximately 1-100 nm in any dimension; the creation anduse of structures, devices, and systems that have novel propertiesand functions because of their small size, and the ability to controlor manipulate matter on an atomic scale.”15The unintentionalformation of nanoparticles thus would not fall under this defini-tion of an engineered nanoparticle. It is estimated that todayabout 320 tons/year of nanosilver are produced and usedworldwide16(data on its historical production are not available).Now what about thefirstreport of nanosilver? Over 120 yearsago, in 1889, M. C. Lea reported the synthesis of a citrate-stabilized silver colloid.17The average diameter for the particlesobtained by this method is between 7 and 9 nm.18Their size inthe nanoscale and the stabilization by citrate are identical torecent reports about nanosilver formation using silver nitrate andcitrate, e.g., refs 19 and 20. Also the stabilization of nanosilverusing proteins has been described as early as 1902.21Under thename“Collargol”such a kind of nanosilver has been manufac-tured commercially since 1897 and has been used for medicalapplications.22Collargol has a mean particle size of 10 nm23andas early as 1907 its diameter was determined to be in thenanorange.24Other nanosilver preparations were also inventedin the next decades, for example the gelatin stabilized silvernanoparticles patented by Moudry in 1953 with 2-20 nmdiameter25and silver nanoparticle impregnated carbon with adiameter of silver particles below 25 nm.26It is important to notethat the inventors of nanosilver formulations understood decadesago that the viability of the technology required nanoscale silver,e.g., by the following statement from a patent:“forproperefficiency, the silver must be dispersed as particles of colloidalsize less than 250 Å [less than 25 nm] in crystallite size”.26Whereas it is true for many other engineered nanomaterials thatthey are novel, e.g., for fullerenes and carbon nanotubes, this is1178dx.doi.org/10.1021/es103316q |Environ.Sci. Technol.2011, 45,1177–1183
Environmental Science & Technology
POLICY ANALYSIS
Table 1. Evaluation of National Pesticide Information Retrieval System (NPIRS; http://ppis.ceris.purdue.edu/npublic.htm)Database for EPA-Registered Silver Productscategoryconfirmed nanolikely nanoionicnot likely nanounknownconclusionnanonanonot nanonot nanonot nanoselection basisevaluation based on public citations stating nano nature of product or direct measurements on productmaterial (e.g., Figures 3 and 4)evaluation based on patent literature and/or manufacturing techniquesproducts contain materials that store and release individual silver ionsproducts contain macro scale materials, e.g., silver-coatedfibersinsufficient information availablenumber of registrations7 (7%)42 (46%)31 (34%)8 (9%)4 (4%)
clearly not the case for nanosilver. This long history of rationalfabrication and use of colloidal nanosilver has resulted in a lot ofresearch and knowledge about these nanoparticles over the last100 years, even if this research is not reported under“nano”terminology.The nanosilver formulations mentioned in the precedingsection have not only been used by scientists and described inthe patent literature, but have consistently found their way intothe market. In the early part of the 20th century, the commercialsale of medicinal nanoscale silver colloids, known under differenttrade names such as Collargol, Argyrol, and Protargol, began andover a 50-year period their use became widespread. Thesenanosilver products were sold as over-the-counter medicationsand also used by medical doctors to treat various diseases such assyphilis and other bacterial infections.27
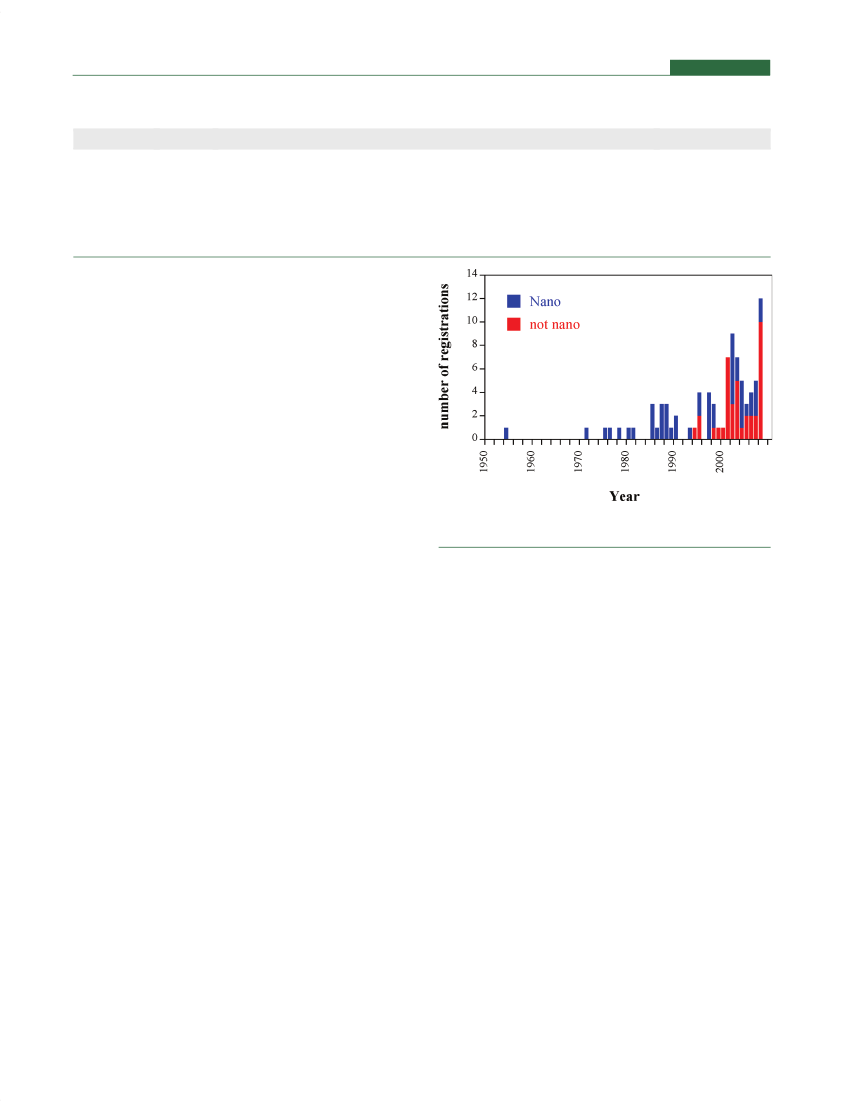
’REGISTRATION OF NANOSILVER PRODUCTS IN THEUNITED STATESApart from these medical applications, many biocidal nano-silver products were developed and registered in the UnitedStates. Information on EPA-registered silver products for the last60 years is contained in the NPIRS database (National PesticideInformation Retrieval System, http://ppis.ceris.purdue.edu/npublic.htm). We searched and evaluated this database for allregistered biocides referring to silver as the active substance, andregistered labels relating to each product were reviewed for theform of the silver and the company name. Additional searches inpublic patent literature and general Internet searches specific tothe company and product were also used to evaluate the natureof each registered product. Based on these reviews the list ofregistered silver products was divided intofivecategories, fromsurely containing“nano”to not likely containing“nano”(Table 1).Of the biocidal silver products, 53% likely or surely containnanosilver but only 7% are advertised as containing nanoparti-cles. For the majority of the registered products only a combinedevaluation of patents and knowledge on manufacturing tech-niques and materials science can inform about the nature of thesilver present in the product. Figure 2 shows the frequency ofEPA-registered silver products over the years. Thefirstbiocidalsilver product registered in the U.S. under the Federal Insecti-cide, Fungicide, and Rodenticide Act (FIFRA) in 1954 wasAlgaedyn, a nanosilver product based on the patent by Moudry25that is still used today as an algicide in residential swimmingpools. After the establishment of EPA in 1970 all silver registra-tions in the next 23 years until 1993 were for nanosilver (colloidalsilver) or for silver nanocomposites. Thefirstnon-nanosilverproduct was registered in 1994. It is also apparent that thenumber of“non-nano”silver products has risen substantially inthe last 10 years, necessitating also new scientific studies onpotential ecotoxicological effects of these“conventional”products.
Figure 2.Registration of biocidal silver and nanosilver products in theU.S. with the categorization according to Table 1.
There are at least three categories of EPA-registered productsthat employ elemental silver particles with particle sizes less than100 nm: (a) silver biocidal additives; (b) silver-impregnatedwaterfilters;(c) silver algicides and disinfectants. Several exam-ples of each category are identified below.Silver Biocidal Additives.EPA has registered numerousbiocidal additives based on elemental silver particles. Table 2contains some examples of currently registered biocidal additiveproducts that contain metallic (elemental) silver with very smallparticle size (<100 nm), including Additive SSB (EPA reg.83587-3, company NanoHorizons), MicroSilver BG-R (EPAreg. 84146-1, company Bio-Gate), and HyGate 4000 (EPA reg.70404-10, company BASF, formerly Ciba Corp.). These silverbiocides are typically used in plastic and textile applicationswhere the silver is effectively contained within polymersubstrates.Silver-Impregnated Water Filters.EPA has registered mul-tiple silver-impregnated water filters since the 1970s. Bacterio-static water filters are generally based on activated carbon orceramics that are impregnated with metallic (elemental) silverexhibiting a very small particle size (<100 nm). It should be notedthat impregnating carbon and ceramic materials with metals iswidely recognized as a standard technique for the synthesis ofnanoscale metal particles. In particular, wet impregnation meth-ods have been employed in production of nanostructuredindustrial catalysts for decades. Numerous peer-reviewed scien-tific literature publications clearly establish that impregnationmethods lead to nanoscale metal particles (e.g., nanosilver)supported on the impregnated substrate (e.g., carbon).28,29Silver-impregnated waterfilterscurrently registered underFIFRA employ impregnation-based manufacturing methods to1179dx.doi.org/10.1021/es103316q |Environ.Sci. Technol.2011, 45,1177–1183
Environmental Science & TechnologyTable 2. Details about Some Selected EPA-Registered Nanosilver Productsproduct typewaterfilterwaterfilteralgicidealgicidealgicideadditiveadditiveadditivea
POLICY ANALYSIS
product name989 Bacteriostatic Water Filter MediaNATURE2 G45-VC40AlgaedynNu-Clo SilvercideASAP-AGXAdditive SSBMicroSilver BG-RHyGate 4000
companyBarnebey & Sutcliffe Corp(Now owned by Calgon)Zodiac Pool Care, Inc.Pool Products Packaging CorpAlden Leeds Inc.American Biotech LaboratoriesNanohorizons Inc.Bio-Gate AGBASF Corp (Formerly Ciba)
EPA registration number58295-167712-168161-17124-10173499-183587-384146-170404-10
date registered1-Dec-198821-Nov-200231-Dec-195415-Jun-199327-Feb-200228-Sep-200718-Mar-20085-Sep-2008
referencea
b
, Figure 3
Figure 4dcdee
US Patent 3,374,608,“SilverImpregnated Carbon” (1968), Pittsburgh Activated Carbon Company, now owned by Calgon (“activatedcarbonimpregnated with a metallic silver having a crystallite size ofnot over 250 A[25 nm].”...“[T]he silver must be dispersed as particles ofcolloidal size (less than250 A”)(emphasis added).bUS Patent 6,165,358,“WaterPurifier for a Spa” (2000). Zodiac Pool Care, Inc.“purificationmaterials are described, forexample, in U.S. Pat. No. 5,352,369”.US Patent 5,352,369,“Methodof Treating Water” (1994). Fountainhead Technologies Inc.:“theelemental silverpreferably includes at least 2% of silver crystals having crystal sizes between approximately3 nmand10 nm”(emphasis added).c“Theseengineered silverparticles currently vary in size between about10-50 nm in diameter”.(April 26, 2005) William D. Moeller, President, American Biotech LaboratoriesTestimony on Malaria before the U.S. House of Representatives, International Relations Committee, Subcommittee on Africa, Global Human Rights,and International Operations. [http://www.foreignaffairs.house.gov/archives/109/20915.pdf] (p.38)].dSNWG“Evaluationof Hazard and ExposureAssociated with Nanosilver and Other Nanometal Oxide Pesticide Products”, Presentation to Scientific Advisory Panel (November 4, 2009). Docket ID:EPA-HQ-OPP-2009-0683-0165. [http://www.regulations.gov/search/Regs/contentStreamer?objectId=0900006480a52512&disposition=attachment-&contentType=pdf].eCiba Specialty Chemicals (Now BASF)“CibaSpecialty Chemicals forms marketing cooperation with Bio-Gate for silver anti-microbial technology” (December 14, 2005, Basel, Switzerland) [http://cibasc.com/index/med-index.htm?reference=41794&checkSum=C441-84952B5155A13ECC5E419C8F7310] Notefigurecaption“Scanningelectron microscopy showing the high porosity HyGate 4000 powder:primaryparticle size 50-200 nm...”(emphasis added).
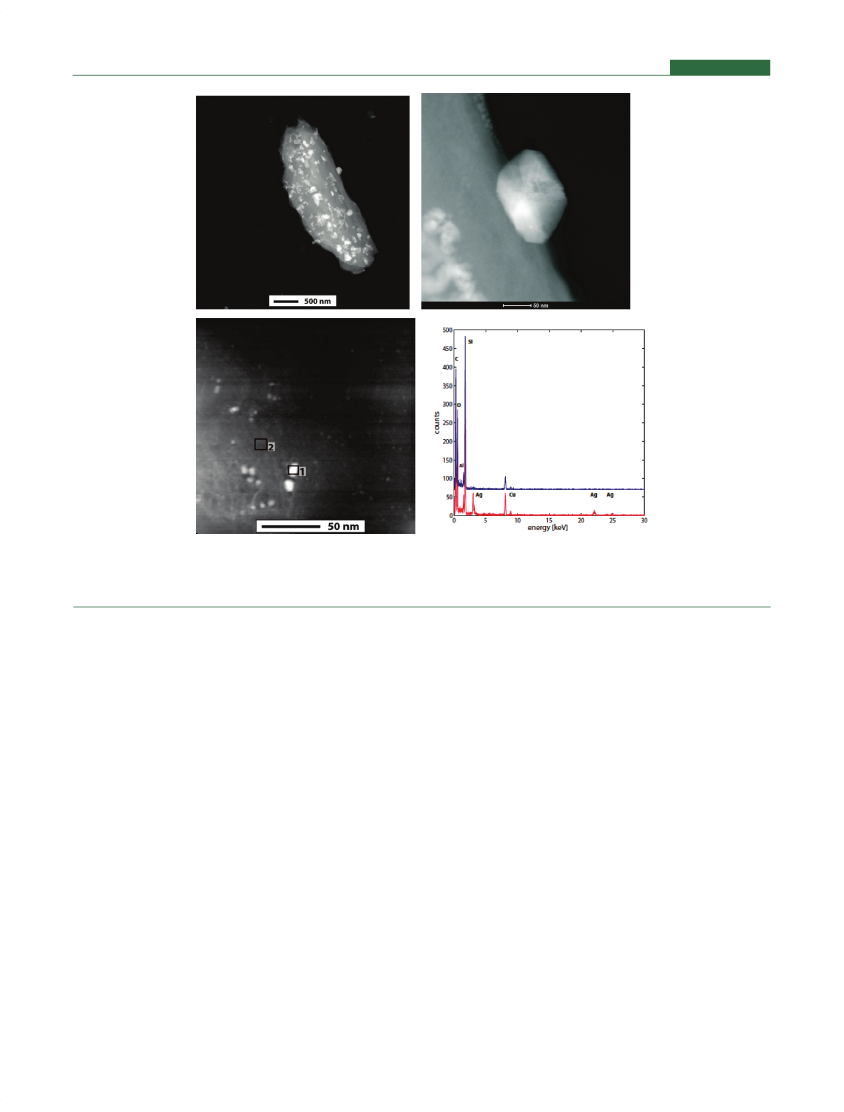
achieve the registered metallic silver form,26,30,31in many caseswith the clear intention to produce nanoscale silver. An exampleis given in one patent application from 1994:“theelemental silverpreferably includes at least 2% of silver crystals having crystal sizesbetween approximately 3 nm and 10 nm”.31Silver-impregnatedwaterfilterscontain nano-silver particles supported on thefiltermatrix structure.To verify the presence of nanoscale Ag in waterfilters,commer-cially availablefilterswere disassembled and the carbon pellets werecrushed and investigated with TEM equipped with a HAADF (HighAngle Annular Dark Field) detector that is sensitive toward heavyelements (Figure 3). Details of the analysis can be found in theSupporting Information. Bright nanoparticles are visible distributedon matrix particles having sizes ranging from a few nm to about 100nm. EDX (energy dispersive X-ray spectroscopy) analysis showedthat the bright particles contain Ag whereas spectra of the light graymatrix background have no detectable Ag signal. These analysesclearly prove that during manufacturing of the silver-impregnatedwaterfiltersAg nanoparticles are formed and that all silver is presentin nanoparticulate form in thesefilters.However, it remains to beinvestigated what the fate of this nanosilver is during use of the waterfilters,whether there is only dissolution and release of ionic silver orwhether there is also release of particulate silver.Silver-impregnated waterfiltershave been safely used fordomestic water applications such as drinking water and swim-ming poolfiltersfor decades. No reports about any health orenvironmental effects have been reported, although the absenceof such reports does not mean that no effects occurred. However,the many decades long use of these EPA registered nanosilvercontaining products in numerous households presents a uniqueopportunity for epidemiologists to investigate effects of extendeduse of nanosilver on human health.Silver Algicides and Disinfectants.Silver algicides anddisinfectants have been FIFRA registered as biocides since
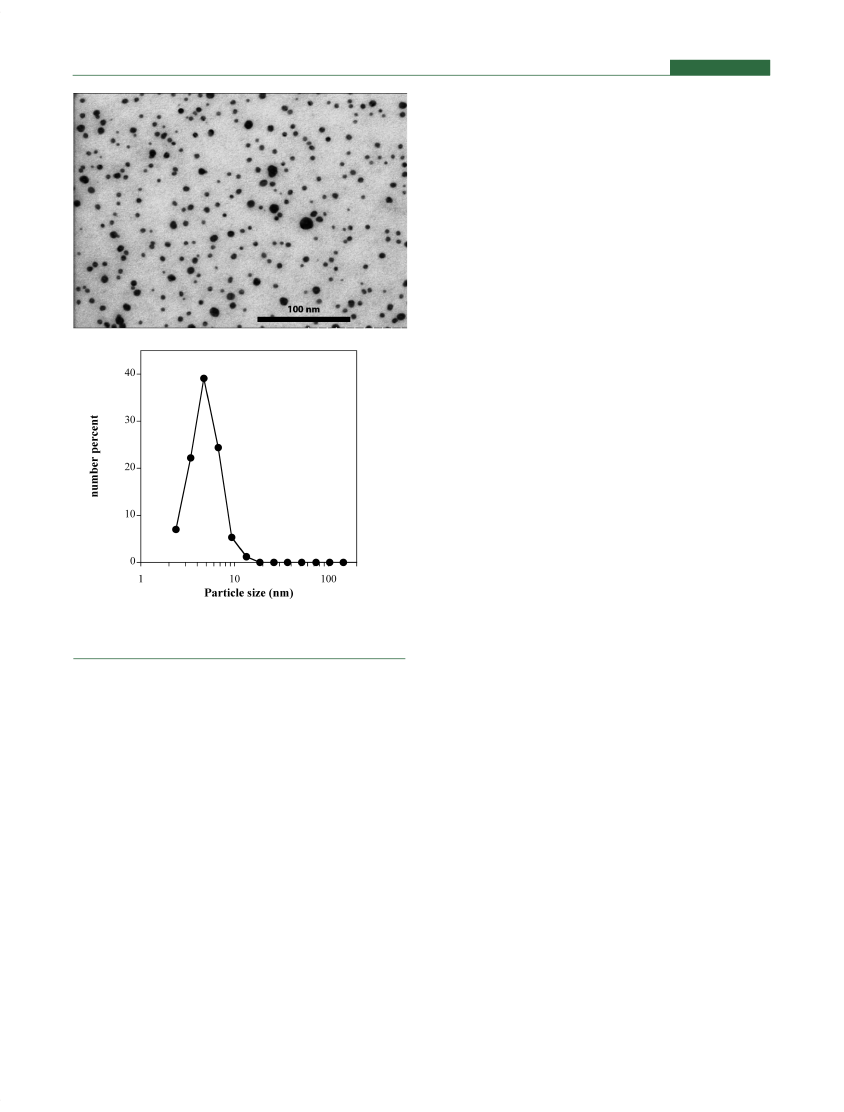
1954. Colloidal nanosilver algicides are based on elemental silverparticles maintained in a stabilized solution, containing silver invery small particle size (e.g., <100 nm). Table 2 contains someexamples of currently registered biocidal products includingSilver Algaedyn (EPA reg. 68161-1, Pool Products PackagingCorp), Nu-Clo Silvercide (EPA reg. 7124-101, Alden Leeds Inc.)and ASAP-AGX (EPA reg. 73499-1, American Biotech Labora-tories). Figure 4 shows a TEM micrograph of Algaedyn and thesize distribution of the particles determined by image analysis(>3500 particles, details of the analysis can be found in theSupporting Information), proving that Algaedyn contains silvernanoparticles with diameters between 2 and 20 nm.It should be noted that algicide applications have been usedsafely in high-exposure, direct water contact, and down-the-drainapplications such as swimming pool disinfection for decadeswithout any known damaging impact on humans or the environ-ment. The more than 50-year use of these nanosilver productspresents a unique opportunity for environmental scientists tostudy the effects of the discharge of nanosilver into sewersystems, wastewater treatment plants, and natural waters, espe-cially in residential areas with a lot of swimming pools. Alsoepidemiologists could study populations of home-owners usingsilver-based algicides and compare with those using otherbiocides to get data on a population that has been exposed tonanomaterials for decades.
’SILVER NANOTOXICOLOGYColloidal nanosilver has been administered as a medication foralmost one hundred years.22Regardless of the medicinal claimsassociated with these products a lot of research on human andanimal body distribution and toxicology was carried out withthese materials. One early example is a study from 1924 in whichthe behavior of Collargol nanosilver in the human body isdescribed.32Numerous cases of the nontoxic, cosmetic condition1180dx.doi.org/10.1021/es103316q |Environ.Sci. Technol.2011, 45,1177–1183
Environmental Science & Technology
POLICY ANALYSIS
Figure 3.TEM analysis of silver-impregnated carbonfilter,Zodiac Nature2 G (EPA registration no. 67712-1). Top left: larger particle with silverparticles, discernible by their brightness; top right: magnification of one silver nanoparticle from the picture to the left; bottom left: small silvernanoparticles on gray matrix; bottom right: EDX spectra of the two areas in the TEM picture on the left (1: silver particles, bottom spectrum,2: background: top spectrum). For details about methods see Supporting Information.
argyria were documented during this period. Argyria is a condi-tion characterized by a bluish-gray discoloration of the skin.33The toxicity of silver is considered to be relatively low3and toxiceffects on humans other than argyria are only observed at veryhigh concentrations (e.g., acute oral LD50 for rats is higher than1600 mg kg-1d-1).3,33Gaul and Staud in 193534listed 43 cases ofargyria of which 27 (63%) were caused by Collargol or Argyrol, thusoriginating from the medical use of colloidal nanosilver. Theother cases were caused by silver chloride or silver iodide. Asignificant portion of the historical toxicological research on theeffects of silver on humans can thus be considered early examples of“nanotoxicology”;predatingwhat is currently considered to benanotoxicology35by more than 80 years.In 1939 Hill and Pillsbury36collected the available literatureon silver and colloidal nanosilver toxicology and derived expo-sure limits based on the threshold value above which develop-ment of argyria can be expected. This threshold value was foundto be the intake of 0.9 g of silver over the whole lifetime. Themodern drinking water standard of 100μg/Lfor silver is basedon this value37and thus includes data on nanoscale-silver. Only avery few studies are available that describe toxicity of bulk silveras opposed to nanosilver or dissolved silver. However, for somestandards there is a distinction between metallic and ionic silver;for instance, the American Conference of Governmental Indus-trial Hygienists has established separate threshold limit values formetallic silver (0.1 mg/m3) and soluble compounds of silver(0.01 mg/m3).33We can thus state that with relation to human
toxicology and the legal standards related to occupational andconsumer health, the toxicity of nanosilver has been taken intoaccount and thus the existing standards are sufficient to protectconsumers also from novel nanosilver, at least in forms equiva-lent to those available in the 1930s.If we turn our attention to environmental risk assessment ofsilver we see a different picture. The data about effects of silver onenvironmental organisms were almost exclusively obtained usingdissolved silver.38Most research on nonmammalian species wasbased on the use of dissolved silver and only recently wereecotoxicological studies with nano-Ag published, e.g., refs 8, 40,and 41. However, it has been questioned if data obtained usingionic silver should be used to derive threshold values in theenvironment.42Silver in natural waters is typically associatedwith the particulate and colloidal fraction43and is thus to someextent naturally present as nanoparticles and metal-sulfideclusters.44Furthermore it should be noted that the latest researchindicates that under real-life environmental exposure conditionsnanosilver is rapidly converted to silver sulfide, resulting inmaterial forms that give no measurable impact on wastewatertreatment plants and are much less toxic than ionic silver.44-46The conversion to silver sulfide highlights the importance ofaccounting for speciation and passivation of silver materials byubiquitous environmental species under real-life conditions inreal-world risk assessment of nanosilver materials.5Because many of the aquatic species are several orders ofmagnitude more sensitive to silver than mammals and humans1181dx.doi.org/10.1021/es103316q |Environ.Sci. Technol.2011, 45,1177–1183
Environmental Science & Technology
POLICY ANALYSIS
Figure 4.TEM analysis of the algicide Algaedyn (EPA registration no.68161-1) (top) and particle size distribution by image analysis(bottom). For details about methods see Supporting Information.
record of demonstrated safe use together with a long period offormal and successful regulatory oversight. The use of very highdoses of colloidal nanosilver at the beginning of the 20th centuryhas sparked a vast amount of research on the toxicology ofnanosilver, resulting in thefirstexposure limits for silver andsubsequent regulations on its use. Clearly nanosilver is a materialthat does notfitthe paradigm of a“new”chemical with new andunknown risks. To consider otherwise is to confuse nomencla-ture (nano) instead of considering the material itself.Regulators rightly state that policy needs to be made on thebasis of sound science. Afirstprinciple of science is thatassumptions need to be tested. For example nanosilver isassumed to be a new material because of the term“nano”.However, on close inspection nanosilver materials have a longhistory of relatively safe and regulated use. Historical perspectivealso shows us that nanosilver has been intentionally manufac-tured and adopted commercially across a wide spectrum of every-day applications for decades. For example, EPA-registered silvernanoparticles have been safely used in down-the-drain and high-volume water-contact applications (e.g., swimming pool algicidesand drinking waterfiltersystems) bringing benefit to millions ofconsumers over a period of 50 years. On balance, a substantialamount is known about silver and silver nanoparticles and thathistorical experience of use and exposure actually points to thesematerials being relatively safe. While there are naturally topicswhere there is ample opportunity to improve scientific under-standing about nanosilver (e.g., with respect to its environmentalbehavior and effects), it would be a mistake for regulators toignore the accumulated knowledge of our scientific and regula-tory heritage in a mistaken bid to declare nanosilver materials asnew chemicals, with unknown properties and automaticallyharmful simply on the basis of a change in nomenclature to theterm“nano”.However, this does also not mean that nanosilvershould be treated as harmless without testing its effect, but ratherthat it is sufficient to apply the already strict and coherent riskassessment framework for other silver-containing materials andproducts that often have a shorter history of regulated use.
(with lethal concentrations for some sensitive aquatic organismsof only 1-5μg/L3), the question whether nanosilver has adifferent toxicity than dissolved silver is of eminent importance.However, this should be discussed in the light of the recentfindingsthat the silver present in the environment is to a largeextent present in particulate form and as nanoclusters and not asdissolved silver.It should also be kept in mind that the expected concentra-tions of nanosilver in the surface waters of the U.S. are between0.09 and 0.43 ng/L47whereas total silver concentrations in waterwere modeled to be between 40 and 320 ng/L for European sur-face waters.42Nanosilver thus contributes only a small extent tothe total silverflowin the environment.
’ASSOCIATED CONTENTSb
TEM analysis of waterfiltersand TEM analysis of Algaedyn. This material is available free ofcharge via the Internet at http://pubs.acs.org.Supporting Information.
’AUTHOR INFORMATIONCorresponding Author
’IMPLICATIONS FOR POLICY OF NANOSILVERRegardless of what nomenclature is used, any concept of riskmust ultimately derive from chemical and physical characteristicsof aspecific material.Applying the general prefix“nano”does notin itself automatically render a material harmful. Although today’snanosilver has many alternative nomenclatures and historicalaliases including“colloidalsilver”, the underlying material is thesame;extremely small particles of silver. Contrary to manycommon assumptions, nanosilver materials have a deep historical
’ACKNOWLEDGMENTWe thank Dr. James Delattre and Dr. Rosalind Volpe of theSilver Nanotechnology Working Group (SNWG) for valuablecontributions to the background of this article. We thank Dr. RalfKaegi from Eawag for performing the TEM analyses.’REFERENCES(1) Nowack, B.; Bucheli, T. D. Occurrence, behavior and effects ofnanoparticles in the environment.Environ. Pollut.2007,150,5–22.(2) Wiesner, M. R.; Lowry, G. V.; Jones, K. L.; Hochella, M. F.; DiGiulio, R. T.; Casman, E.; Bernhardt, E. S. Decreasing Uncertainties inAssessing Environmental Exposure, Risk, and Ecological Implications ofNanomaterials.Environ. Sci. Technol.2009,43(17), 6458–6462.1182dx.doi.org/10.1021/es103316q |Environ.Sci. Technol.2011, 45,1177–1183
Environmental Science & Technology(3) Wijnhoven, S. W. P.; Peijnenburg, W. J. G. M.; Herberts, C. A.;Hagens, W. I.; Oomen, A. G.; Heugens, E. H. W.; Roszek, B.; Bisschops,J.; Gosens, I.; Van De Meent, D.; Dekkers, S.; De Jong, W. H.; vanZijverden, M.; Sips, A. J. A. M.; Geertsma, R. E. Nano-silver - A review ofavailable data and knowledge gaps in human and environmental riskassessment.Nanotoxicology2009,3(2), 109–138.(4) Scheringer, M.; MacLeod, M.; Behra, T.; Sigg, L.; Hungerb€hler,uK. Environmental risks associated with nanoparticulate silver used asbiocide.Household Pers. Care Today2010,1,34–37.(5) Nowack, B. Nanosilver revisited downstream.Science2010,330,1054–1055.(6) Benn, T. M.; Westerhoff, P. Nanoparticle silver released intowater from commercially available sock fabrics.Environ. Sci. Technol.2008,42(11), 4133–4139.(7) Geranio, L.; Heuberger, M.; Nowack, B. Behavior of silver nano-textiles during washing.Environ. Sci. Technol.2009,43,8113–8118.(8) Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.;Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles toChlamydomonas reinhardtii. Environ. Sci. Technol.2008,42(23),8959–8964.(9) Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.;Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticlesfrom outdoor facades.Environ. Pollut.2010,158(9), 2900–2905.(10) Bell, F. A. Review of effects of silver-impregnated carbonfilterson microbial water quality.J. Am. Water Works Assoc.1991,83(8),74–76.(11) Adamson, R. P.; Sommerfeld, M. R. Laboratory comparison ofthe effectiveness of several algicides in isolated swimming pool algae.Appl. Environ. Microbiol.1980,39(2), 348–353.(12) Egger, S.; Lehmann, R. P.; Height, M. J.; Loessner, M. J.;Schuppler, M. Antimicrobial Properties of a Novel Silver-Silica Nano-composite Material.Appl. Environ. Microbiol.2009,75(9), 2973–2976.(13) Kumar, R.; Howdle, S.; Munstedt, H. Polyamide/silver anti-microbials: Effect offillertypes on the silver ion release.J. Biomed. Mater.Res. B2005,75B(2), 311–319.(14) Aruguete, D. M.; Hochella, M. F. Bacteria-nanoparticle inter-actions and their environmental implications.Environ. Chem.2009,7(1), 3–9.(15) EPA.Nanotechnology White Paper;Report EPA 100/B-07/001;U.S. Environmental Protection Agency: Washington, DC, 2007.(16) Gottschalk, F.; Scholz, R. W.; Nowack, B. Probabilistic materialflowmodeling for assessing the environmental exposure to compounds:Methodology and an application to engineered nano-TiO2particles.Environ. Modeling Software2010,25,320–332.(17) Lea, M. C. On allotropic forms of silver.Am. J. Sci.1889,37,476–491.(18) Frens, G.; Overbeek, J. T. Carey Leas colloidal silver.Kolloid Z.Z. Polym.1969,233(1-2), 922–&.(19) Henglein, A.; Giersig, M. Formation of colloidal silver nano-particles: Capping action of citrate.J. Phys. Chem. B1999,103(44),9533–9539.(20) Dong, X. Y.; Ji, X. H.; Wu, H. L.; Zhao, L. L.; Li, J.; Yang, W. S.Shape Control of Silver Nanoparticles by Stepwise Citrate Reduction.J. Phys. Chem. C2009,113(16), 6573–6576.::(21) Paal, C. Uber colloidales Silber.Ber. Dtsch. Chem. Ges.1902,35(2), 2224–2236.::(22) Boese, K. Uber Collargol, seine Anwendung und seine Erfolgein der Chirurgie und Gyn€kologie.Dtsch. Z. Chir.1921,163(1-2),a62–84.(23) Bogdanchikova, N. E.; Kurbatov, A. V.; Tret’yakov, V. V.;Rodionov, P. P. Activity of colloidal silver preparations towards smallpoxvirus.Pharm. Chem. J.1992,26(9-10), 778–779.(24) Bechhold, H. Die Gallertfiltration.Z. Chem. Ind. Kolloide1907,2,3-9–33-41.(25) Moudry, Z. V.Process of producing oligodynamic metal biocides.United States Patent 2,927,052, 1953.(26) Manes, M.Silver impregnated carbon.United States Patent3,374,608, 1968.
POLICY ANALYSIS
(27) Fung, M. C.; Bowen, D. L. Silver Products for MedicalIndications: Risk-Benefit Assessment.Clin. Toxicol.1996,34(1),119–126.(28) Kasemo, B.; Johansson, S.; Persson, H.; Thorm€hlen, P.;aZhdanov, V. P. Catalysis in the nm-regime: Manufacturing of supportedmodel catalysts and theoretical studies of the reaction kinetics.Top.Catal.2000,13,45–53.(29) Ryu, S. K.; Eom, S. Y.; Cho, T. H.; Edie, D. D. Distribution ofSilver Particles in Silver-containing Activated Carbon Fibers.Carbon Sci.2003,4(4), 168–174.(30) Kumar, V. S.; Nagaraja, B. M.; Shashikala, V.; Padmasri, A. H.;Madhavendra, S. S.; Raju, B. D.; Rao, K. S. R. Highly efficient Ag/Ccatalyst prepared by electro-chemical deposition method in controllingmicroorganisms in water.J. Mol. Catal A: Chem.2004,223(1-2), 313–319.(31) Heinig, C. F.Method of treating water.United States Patent5,352,369, 1994.::(32) Schlee, H.; Zweifel, E. Uber das Verhalten von Silberpr€paraten,ainsbesondere von Kollargol im Organismus.Z. Hyg. Infekt.1924,102,454–460.(33) Drake, P. L.; Hazelwood, K. J. Exposure-Related Health Effectsof Silver and Silver Compounds: A Review.Ann. Occup. Hyg.2005,49(7), 575–588.(34) Gaul, L. E.; Staud, A. H. Seventy cases of generalized argyriafollowing organic and colloidal silver medication, including biospectro-metric analysis of ten cases.J. Am. Med. Assoc.1935,104(16), 1387–1390.(35) Oberd€rster, G.; Oberd€rster, E.; Oberd€rster, J. Nanotoxicol-oooogy: An emerging discipline evolving from studies of ultrafine particles.Environ. Health Perspect.2005,113(7), 823–839.(36) Hill, W. R.; Pillsbury, D. M.Argyria, the Pharmacology of Silver;The Williams & Wilkins Co.: Baltimore, MD, 1939.(37) Purcell, T. W.; Peters, J. J. Historical impacts of environmentalregulation of silver.Environ. Toxicol. Chem.1999,18(1), 3–8.(38) EPA. Registration eligibility document Silver, List D, Case4082; U.S. Environmental Protection Agency: Washington, DC, 1992.(39) Shouse, S. S.; Whipple, G. H. Effects of intravenous injection ofcolloidal silver upon the hematopoietic system in dogs.J. Exp. Med.1931,53(3), 413–420.(40) Lee, K. J.; Nallathamby, P. D.; Browning, L. M.; Osgood, C. J.;Xu, X. H. N. In vivo imaging of transport and biocompatibility of singlesilver nanoparticles in early development of zebrafish embryos.ACSNano2007,1(2), 133–143.(41) Choi, O.; Hu, Z. Q. Size dependent and reactive oxygen speciesrelated nanosilver toxicity to nitrifying bacteria.Environ. Sci. Technol.2008,42(12), 4583–4588.(42) Blaser, S. A.; Scheringer, M.; MacLeod, M.; Hungerbuhler, K.Estimation of cumulative aquatic exposure and risk due to silver:Contribution of nano-functionalized plastics and textiles.Sci. TotalEnviron.2008,390(2-3), 396–409.(43) Shafer, M. M.; Overdier, J. T.; Armstong, D. E. Removal,partitioning, and fate of silver and other metals in wastewater treatmentplants and effluent-receiving streams.Environ. Toxicol. Chem.1998,17(4), 630–641.(44) Luther, G. W.; Rickard, D. T. Metal sulfide cluster complexesand their biogeochemical importance in the environment.J. Nanopart.Res.2005,7(4-5), 389–407.(45) Choi, O.; Cleuenger, T. E.; Deng, B. L.; Surampalli, R. Y.; Ross,L.; Hu, Z. Q. Role of sulfide and ligand strength in controlling nanosilvertoxicity.Water Res.2009,43(7), 1879–1886.(46) Kim, B.; Park, C.-S.; Murayama, M.; Hochella, M. F. Discoveryand Characterization of Silver Sulfide Nanoparticles in Final SewageSludge Products.Environ. Sci. Technol.2010,44,7509–7514.(47) Gottschalk, F.; Sonderer, T.; Scholz, R. W.; Nowack, B.Modeled environmental concentrations of engineered nanomaterials(TiO2, ZnO, Ag, CNT, fullerenes) for different regions.Environ. Sci.Technol.2009,43,9216–9222.1183
dx.doi.org/10.1021/es103316q |Environ.Sci. Technol.2011, 45,1177–1183