Sundhedsudvalget 2010-11 (1. samling)
SUU Alm.del Bilag 33
Offentligt
SCIENCE POLICY BRIEFING • September 2010
40
Male Reproductive HealthIts impacts in relation to generalwellbeing and low European fertility rates
ContentsForeword
1•Foreword1•Introduction2•Issues and challenges
7•Conclusions8•Recommendations for acommon research strategy
9•References12•Expert Group12•Acknowledgement
Research in the area of male reproductive health has in thepast focused mainly on birth control and family planningin non-developing countries, contraception, and sexuallytransmitted diseases such as HIV. Only little attentionhas been paid to male reproductive health disordersthat lead to impaired fertility resulting in lower birth ratesespecially in industrialised countries. There is thereforean urgent need for better understanding the status ofmale reproductive health, especially in Europe and inindustrialised countries where lifestyle and environmentalfactors may have a negative impact.This Science Policy Briefing is thefirstto highlightthis important issue which could have a dramaticimpact on future birth rates and demographic changesin industrialised countries. It summarises the variousexogenous and endogenous factors which can have animpact on male reproductive health and provides policyadvice to national and European funding institutions.The report was developed by a group of leadingEuropean experts. The issue wasfirstraised by ProfessorNiels E. Skakkebæk during a mini symposium organised atthe European Medical Research Councils (EMRC) plenarymeeting in Strasbourg in April 2009. Afirststrategicmeeting was held in Copenhagen on 20 May 2009 andthe report was then written andfinalisedby the high levelexpert group present at this meeting.This paper aims to increase awareness about the majorconsequences that reduced male reproductive healthcan have. It also provides advice on where and how tostrengthen research in this area. Male reproductive healthhas been a low priority for funding agencies in Europeancountries over the last 25 years. This has led to a lack ofcontinuity in funding and a large translational gap betweenbasic scientists and clinicians working with Europeanpatients.••••The main policy recommendations are as follows:Increase awareness of male reproductive healthissuesStrengthen interdisciplinary, translational research inthe area of male reproductive health issuesImplement long-term, epidemiological studies aimed atbetter understanding the causes and effects of malereproductive disordersTarget research efforts at preventing/minimising theoccurrence of disorders rather than developing drugtreatments.
Recommended funding instruments are transdisciplinaryresearch networks which should be implemented at theEuropean and international level to strengthen this highlyimportant research area for the benefit of society.We would like to thank the members of the high levelexpert group for their excellent work.Professor Marja Makarow,ESF Chief ExecutiveProfessor Liselotte Højgaard,EMRC Chair
IntroductionIn most European countries fertility rates have declineddrastically to below replacement level – the level at whichthe rate of new births can replace a population (1,2). Thisdecline is primarily due to changes in social and economicconditions, such as wider use of contraception and morewomen seeking careers and postponing childbirth (1).However, declining fertility rates may also partly result froma decreased ability to conceive. In Europe there is a grow-ing demand for use of assisted reproduction techniques(ART; 3,4), and a growing body of evidence points towardsadverse trends in male reproductive health, includingreduced semen quality, increased incidence of testicularcancer and increased or an already high incidence of con-genital reproductive malformations (cryptorchidism andhypospadias; 5). It is to be expected that poor semen qual-ity in young men, when combined with the high prevalenceof increased age at attempting for pregnancy in women(when fertility is already declining), will lead to increasedfertility problems in couples and its attendant socio-eco-nomic impacts.Other than cancers, reproductive problems in men aregenerally not life-threatening, but in the lastfiveyears therehas been a growing recognition that male reproductivefunction and risk of cardiometabolic disorders, includingabdominal obesity, type 2 diabetes and hypertension areinterlinked, as late-onset hypogonadism (low/subnormaltestosterone levels) in men is an important determinantand/or consequence of these disorders (6,7). Moreover,the (normal) age-related decline in testosterone levels inmen (8) clearly predisposes to such disorders with broadeffects on wellbeing and mortality (7,9). Estimates of theincidence of hypogonadism vary from ~10% (10) to nearer40% in men >45 years (11). The European-wide increase inthe proportion of the male population that are of older age
www.esf.org
thus carries with it the prospect of an increasing propor-tion of men with hypogonadism, and thus a progressiveincrease in prevalence of cardiometabolic disorders inthe male population, irrespective of any change in dietand exercise. However, perhaps more worrying is theevidence that these problems may also be emerging inmuch younger men. Thus, large studies in both Europeand the US document a trend for declining testosteronelevels in men (of any age) according to more recent yearof birth (12,13), and have shown a clear negative correla-tion between visceral fat levels and lower testosteronelevels (14). At present, it is not clear to what extent it isabdominal obesity that is causing lower testosteronelevels and to what extent it is the other way around. Themost likely scenario, especially in relation to aging, isthat it is a ‘vicious circle’. Thus, more research is neededto better understand these mechanisms.Based on the issues described above, there are cogentreasons for concern about the remarkably poor state ofmale reproductive health across Europe. Not only doesthis have implications for population maintenance andreplacement, but it also augurs for more pervasive andmore life-threatening changes in men’s cardiometabolichealth, a change that may not just be restricted to theaging population. These changes pose hugefinancialand healthcare issues for European governments. Thereis therefore an urgent need for implementation of a com-mon research strategy to better understand the statusof male reproductive health in Europe and the causesof its problems and its inter-relations with wider healthissues. This is the focus of this report.
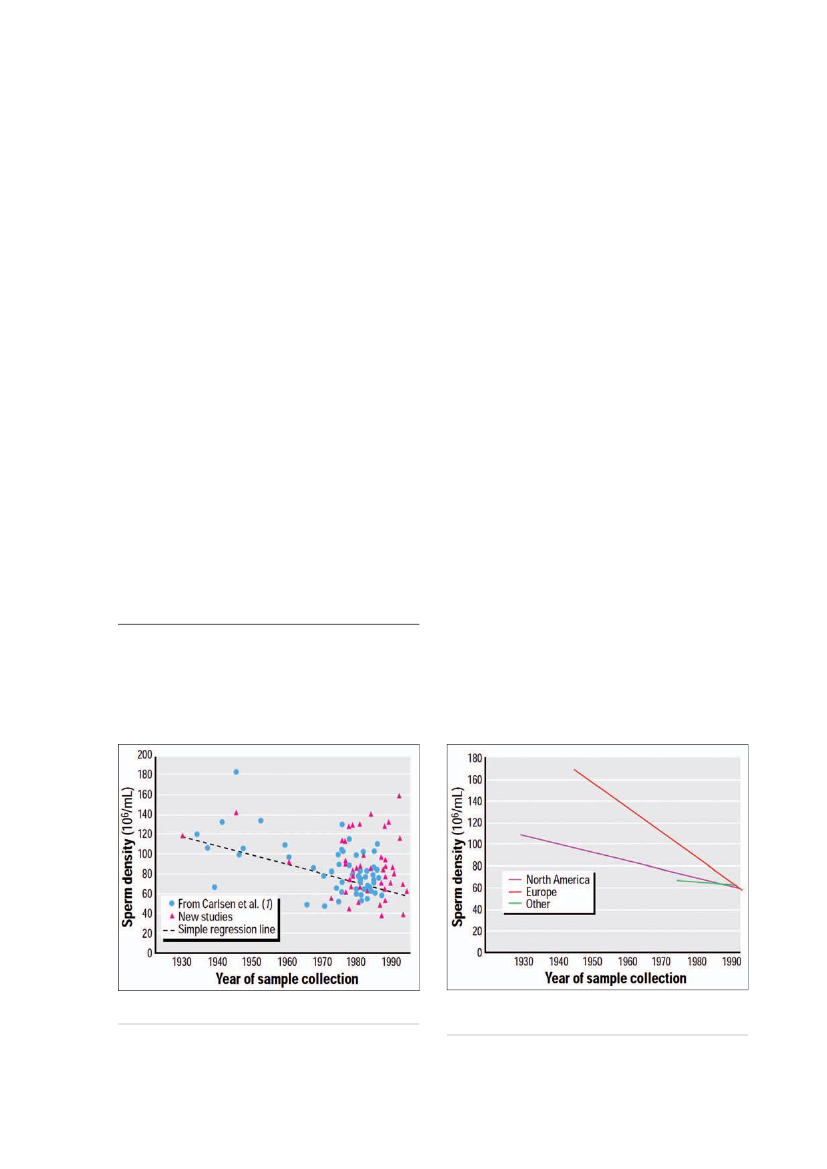
from 113×106/ml to 66×106/ml; 15). There has been a lot ofdiscussion about these results and different attempts toreanalyse the data within the scientific community (16-18;Figs. 1 and 2). Nevertheless, the question of temporalchanges in semen quality still remains controversial,and there are reports of unchanged or even increasingsemen quality in some regions (17). However, recentprospective investigations have, in accordance with thereported adverse trend, found a remarkably poor semenquality among young men from general populations inNorthern Europe (18,19). Approximately 20% of youngmen in various European countries had a sperm concen-tration below the lower WHO reference level (<20x106sperm/ml) and 40% of the men had a sperm concen-tration below the level that has been associated withprolongation of the waiting time to pregnancy (40x106/ml; 20). These trends in semen quality may also havewider implications for health in general, as men with poorsemen quality seem to have increased mortality ratesand shorter life expectancy (21).Worldwide studies of fertile men using standardisedprotocols have shown significant regional differencesin semen quality (22-24). Finnish (Turku) men have a35% higher sperm concentration than do Danish men,while Scottish and French men have sperm counts inbetween these extremes (22). Similar regional differencesin semen quality were found between fertile men fromdifferent US cities (23). Japanese fertile men had a spermconcentration at the same low level as Danish men (24)and men from Singapore had even lower concentra-tions (25). The reasons for these significant geographicaldifferences in semen quality are largely unknown andshould be further examined. Similar regional differencesin other disorders of the male reproductive system havebeen observed, including testicular germ cell cancer(TGC) and congenital malformations of the male repro-ductive tract (5).One reason for discrepancies in the results of semenquality studies could be insufficient quality managementsystems in different geographic areas which may affectthe validity of the results. To assure comparability of
Issues and challengesDeclining semen qualitySemen quality has been declining throughout the pasthalf century in industrialised countries (15,16). Studiesindicate a significant ~50% decrease in semen quality inmen without fertility problems (dropping sperm counts
Figure 1.Mean sperm density in 101 studies published between1934 and 1996 and simple regression line (from ref. 16).
Figure 2.Interactive regression model for mean sperm densityby year and geographic region after controlling for proven fertility(from ref. 16).
2|Science Policy Briefing 40 – Male Reproductive Health – September 2010
all endpoints of semen analysis, the WHO LaboratoryManual for the Examination of Human Semen andSperm-Cervical Mucus Interaction provides a basisfor global standards. To verify high standards, qualitymanagement systems for semen analysis have beenimplemented by various Andrology Societies in sev-eral European countries (e.g. QuaDeGA, Germany; UKNEQAS Andrology; EQA programme, ESHRE). Thesequality control systems have been running success-fully for years and provide a good basis and trainingfor all participants to harmonise and maintain a highstandard of analysis. It will be important for thefieldto maintain quality control programmes and to extendthese schemes throughout Europe.
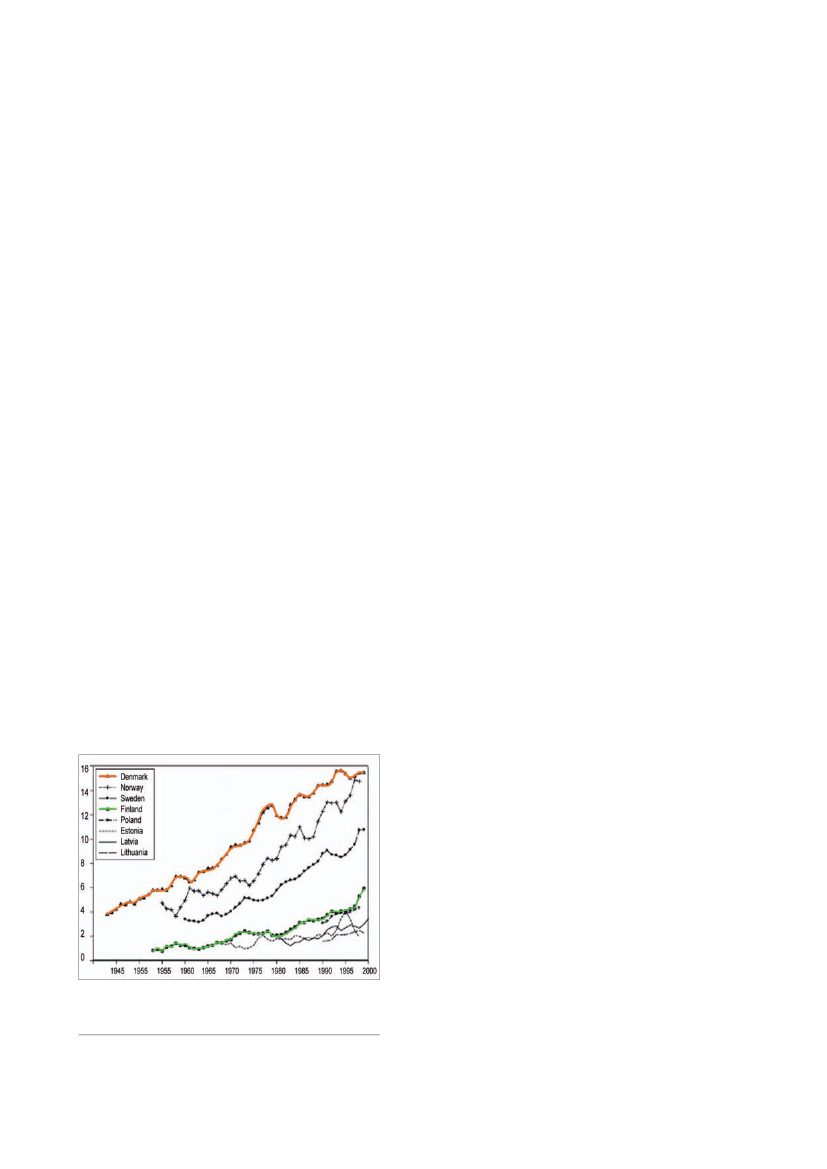
Testicular germ cell cancer (TGC)TGC is the commonest cancer in young men in manycountries. It is well documented to be associated withimpaired semen quality (26) and lower fertility rates, evenprior to development of the cancer (27). The incidenceof TGC has been increasing over the past 40 to 50 yearsin the majority of industrialised countries (28-30; Fig.3)coincident with the declining trend in semen quality.The aetiology of TGC is unknown, but there is abundantevidence that cancerin situof the testis, which is aprecursor for TGC, is generated during fetal develop-ment and TGC therefore has a prenatal origin (31,32).The regional differences in TGC incidence in Europefollow the same pattern as observed for semen quality,as semen quality in high-risk TGC areas is lower thanin low-risk TGC areas (33). As an example, studies inDenmark and Finland indicate that the age-standardisedincidence rates of TGC in 1995 were 15.4 per 105and3.1 per 105, respectively, following the pattern of lowersemen quality among Danish men compared to Finnishmen. These studies have to be expanded at Europeanlevel to better understand these results.
the penis) are among the most common congenitalmalformations in human males. These two congeni-tal abnormalities share common risk factors (34,35)and are both associated with reduced fertility (36,37).Cryptorchidism is also associated with poor semen qual-ity (36) and a considerably increased risk of TGC (38). Theincidence of these malformations appears to have beenincreasing in the Western world over recent decades,with an apparent levelling off in hypospadias incidencein most European countries during the 1980s (39). Atpresent there is only a limited number of studies avail-able. Recent prospective, cohort studies in Denmark andin the UK indicate that the incidence of cryptorchidism atbirth may be far higher than had been supposed (40,41)although a much lower incidence was found in Finland.A similar difference in the incidence of hypospadias wasalso found between Denmark and Finland (42). Thus,the geographic difference in incidence of both cryp-torchidism and hypospadias parallels the pattern forTGC and semen quality in these countries. Much moreresearch is needed to better understand these healthproblems and their relationships.
Fertility and fecundityThe crucial question is whether semen quality amongyoung men in Europe is now so low that it has reacheda threshold at which fertility rates may be affected. In arecent study of pregnancy rates among native Danishwomen born between 1960 and 1980 (43), a ‘total naturalconception rate’ (TNCR) was calculated, which includedboth the total number of births and induced abortions,and excluded births after the use of ART. Among theyounger cohorts, who had notfinishedtheir reproductivecareer, projections were used to estimate their futurefertility. Younger Danish cohorts of women had progres-sively lower TNCR, while the use of ART substantiallyincreased, partly compensating for the decline in TNCR.The results suggest a cohort-related decline in fecundity(ability to conceive). Due to the partly prognostic natureof the study the results are, however, hedged with adegree of uncertainty, and new studies including themost recent registry data will be informative to exam-ine the precision of the projections. On the other hand,thefindingsare consistent with a growing demand forART in Denmark. It has been estimated that more than7% of all children born in 2007 in Denmark were con-ceived by use of ART, which includesin vitrofertilisation,intracytoplasmic sperm injection (ICSI), and intrauterineinsemination (44). Poor semen quality may be part ofthe reason for the increasing use of ART, which is con-firmedby the increasing use of ICSI. Dependency on ARTwould dramatically influence society, since only limitedresources are available for state-supported healthcareand those who do not qualify to receive free ART haveto pay for the possibility to have children. The high costsof ART will certainly put people in an unequal positionfor their chances to conceive. Thus more research atinternational level is needed to provide information fromother countries and to implement a common strategy toimprove the situation.
Congenital malformations(see also TDS section below)Cryptorchidism (undescended testis) and hypospa-dias (incomplete fusion of the urethral folds that form
Figure 3.Trends in incidence of testicular cancer in NorthernEurope. Age-standardised (world standard population) incidenceof testicular cancer by year of diagnosis, country and histologicaltype (from ref. 30).
Science Policy Briefing 40 – Male Reproductive Health – September 2010|3
Declining serum testosterone levels in American Men
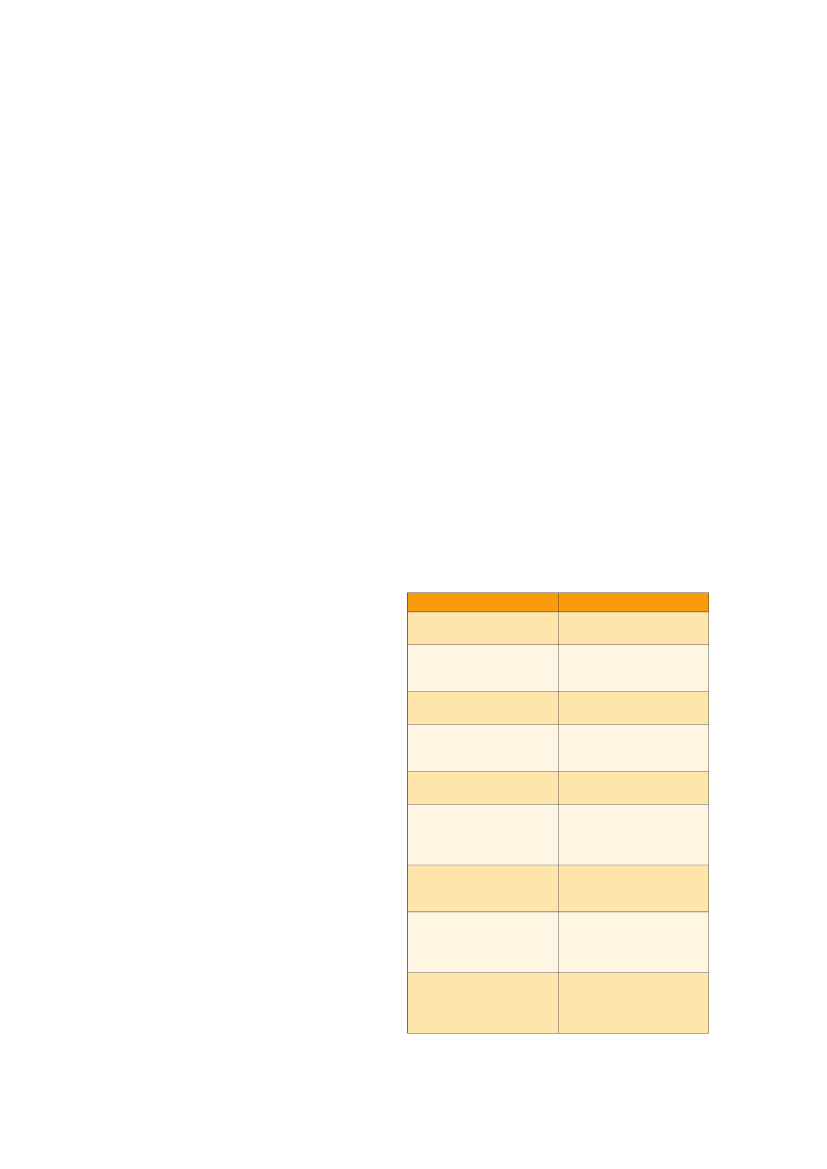
Testicular dysgenesis syndrome (TDS)In Europe there has been a synchronised upward trendin incidence of TGC and congenital reproductive tractmalformations at the same time as a downward trendin semen quality and testosterone levels (although thereare only data for the latter in Denmark). In addition, mostof these disorders share common risk factors and arerisk factors for each other. It has been proposed (5) thatthese conditions may represent a syndrome of disorders,a testicular dysgenesis syndrome (TDS; Fig. 5) causedby a common underlying entity, which results in a dis-turbance of the development of the testes during fetallife. Resulting from TDS one or more of the followingsymptoms may occur: cryptorchidism, hypospadias,decreased spermatogenesis and TGC. The aetiology ofTDS is unclear, but the apparent rapid increase in malereproductive health problems during a few generationssuggests that changes in lifestyle and/or in environmentalfactors are more likely causes than genetic factors (seesections above).
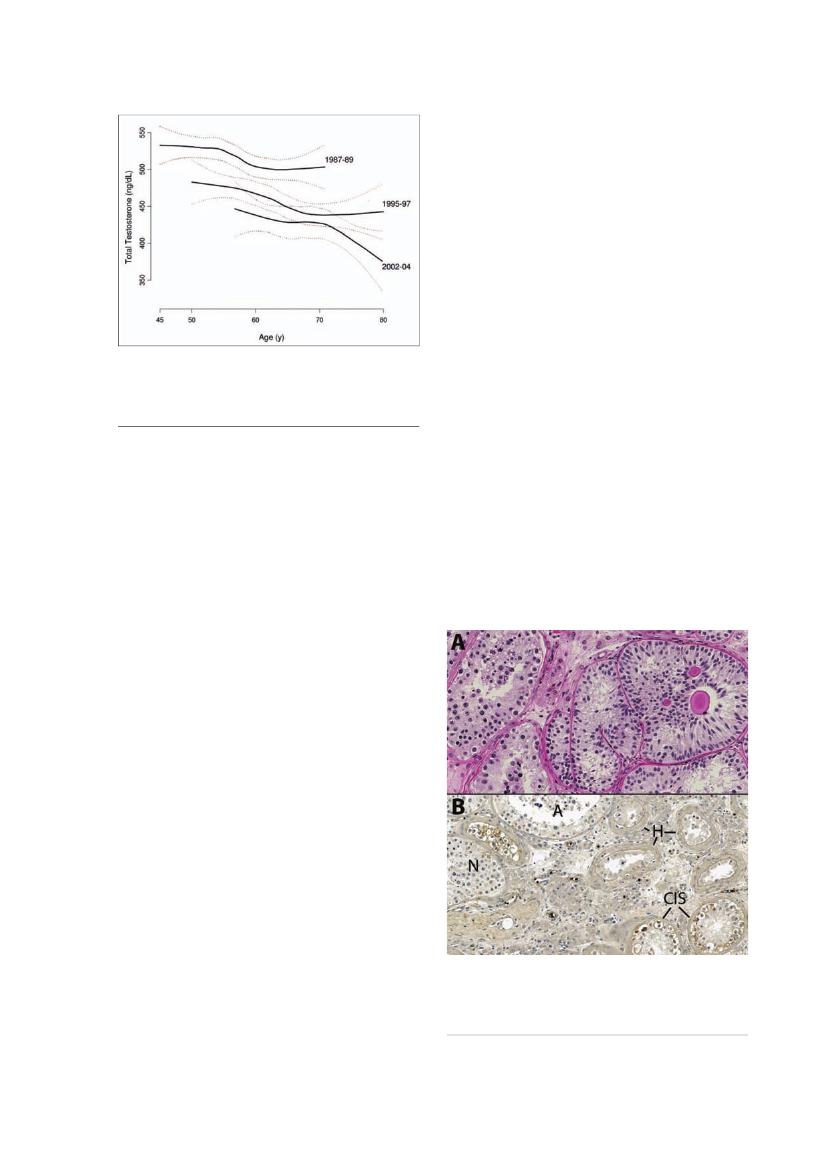
Figure 4.A substantial age-independent decline in testosteronethat did not appear to be attributable to observed changes inexplanatory factors, including health and lifestyle characteristicssuch as smoking and obesity. The estimated population leveldeclines were greater in magnitude than the cross sectionaldeclines in testosterone typically associated with age (from ref. 12).
Endocrine disrupting chemicals (EDC)Testosterone levelsTestosterone is the major driver of male reproductivedevelopment and function and suppression of its levelswithin the adult testis shuts down spermatogenesis (theprocess by which mature sperm cells are formed) andinduces infertility. Testosterone levels within the testisare around 200-fold higher than in peripheral blood.However, lower intratesticular testosterone levels cansustain spermatogenesis. Studies of men with idiopathicinfertility and low sperm counts often show evidence forabnormal function of Leydig cells (cells that producetestosterone) when compared with normospermic fer-tile men, such that their blood testosterone levels areeither low or show evidence of ‘compensated failure’ – asituation in which increased luteinising hormone driveto the Leydig cells is required to maintain testosteronelevels within the normal range (45,46). It is suspected,but unproven, that such compensation will predisposeto more overt Leydig cell failure during aging (46), with itsattendant health consequences, as outlined above.The fact that across Europe the prevalence of oligo-zoospermia (low sperm numbers) in young men (18-25years) is of the order of 20% (see above) could suggestthat the prevalence of Leydig cell dysfunction in thispopulation may also be high or may occur with highfrequency as the men begin to age, thus predisposingthem to cardiometabolic disease. Abdominal obesityis clearly associated with reduced testosterone levels(6,14) and it is also established that obesity (BMI >25) isassociated with an approximate 20% reduction in spermcounts (47), although it is not clear if it is the obesitythat causes the low sperm counts or whether there isan underlying common cause for both conditions. Asmentioned earlier, studies in both Denmark and the USindicate a birth cohort-related decline in testosteronelevels in men (12,13; Fig.4), echoing the similar declinein sperm counts.EDC are exogenous substances that alter one or morefunctions of the endocrine system and consequentlycause adverse health effects in an intact organism, orits progeny, or (sub)populations (WHO, InternationalProgramme on Chemical Safety, IPCS).Scientific focus has in particular been directed towardsEDC as possible contributing factors to the rise in inci-dence of TDS disorders (49). EDC have the potentialcapability of interfering with the sexual organs in earlyfetal life. The process of a fetus developing into a male
Figure 5.Testicular specimens from patients with TDS. A) Infertileman. Note abnormal spermatogenesis (left) and tubules containingonly undifferentiated Sertoli cells and intratubular microliths (right).B. Mixed pattern with normal (N) and abnormal (A)spermatogenesis, hyalinised tubules (H) and tubules withcarcinomain situ(CIS).
4|Science Policy Briefing 40 – Male Reproductive Health – September 2010
involves a complex cascade of events. This is initiatedby sex-determining genes, which activate the processof testis formation, which is a hormone-independentprocess. In contrast, subsequent steps in masculinisa-tion, which include formation of the external genitaliaand descent of the testes into the scrotum, are hor-mone-dependent (48). Three hormones are involved,anti-Müllerian hormone, testosterone and insulin-likefactor 3, but of these testosterone (an androgen) has thewidest ranging effects. Androgens are responsible formasculinisation of the external genitalia andfinaltestisdescent into the scrotum, events which are programmedor induced during thefirsttrimester of pregnancy, sothe timing of testosterone secretion is critical for normaldevelopment of the reproductive organs. Impairmentof action of androgens in a male fetus leads to under-masculinisation, while exposure of a female fetus toandrogens will cause masculinisation (49). EDC withanti-androgenic and estrogenic (e.g. diethylstilboestrol)and possibly other properties may therefore potentiallydisturb the development of reproductive organs duringfetal life.Animal experiments have shown that certain EDC cancause adverse effects in the male reproductive systemthat resemble the disorders described in human TDS,except for TGC (50). Wildlife exposed to environmen-tal contaminants also exhibit abnormal reproductivedevelopment (51). The list of chemicals that have beenidentified as having endocrine disrupting properties inanimal studies is growing and includes numerous sub-stances found in household and consumer products; e.g.phthalates in many domestic, commercial and personalcare products, and dioxin infishand milk products (seeexamples in Table 1).The mechanisms via which synthetic chemicals affecthormone action during masculinisation are only knownfor a few compounds. Some substances have beenidentified as being anti-androgenic because they bindto, but do not activate, the androgen receptor (AR), e.g.p,p’-DDE, which is a metabolite of the pesticide DDT,and the fungicide vinclozolin (52). In contrast, certainphthalate esters (e.g. diethylhexyl phthalate and di-n-butylphthalate) interfere with androgen biosynthesisin the fetal testis, resulting in anti-androgenic effects(49). Other chemicals exhibit estrogenic activity, and theadverse effects of estrogens in male animals are to anextent similar to those of anti-androgens (49). An exam-ple of an estrogenic chemical is bisphenol A, which in the1930s was identified as a weak synthetic estrogen (53).Bisphenol A exerts estrogenic effects through binding toestrogen receptors (54,55) but it may also exert effectsthat are not estrogen-mediated. Some chemicals can actthrough multiple mechanisms, for example the fungicideprochloraz, which acts both by blocking the AR and byinhibiting fetal androgen production (56).The effects of EDC are usually studied in animals at(maternal-fetal) exposure levels higher than those towhich humans are typically exposed. However, in severalstudies exposure to mixtures of between three and sevenchemicals with anti-androgenic properties, at doses atwhich each chemical alone was without significant effect,caused major impairment of masculinisation and occur-
rence of hypospadias (57,58). As humans are exposedto a complex cocktail of environmental chemicals (59),it is assumed that similar additive effects will also occur.This being the case, it introduces enormous complexityto identifying the causal contribution to TDS disordersof individual chemicals. The administrative regulation ofsuch chemicals presents similar complexity (60).Some human studies have found associationsbetween exposure to EDC and malformations of themale urogenital tract. Higher concentrations of persist-ent pesticides (61) andflameretardants (62) in humanbreast milk as well as maternal occupational pesticideexposure early in pregnancy have also been found tobe related to increased risk of cryptorchidism amongthe offspring (63).Few studies have examined the effects of prenatalexposure to EDC on future semen quality and risk of tes-ticular cancer, probably due to the challenging lag timebetween exposures and the occurrence of these disor-ders, which do not manifest until after puberty (64).The epidemiological evidence of current exposureto EDC on semen quality is also still sparse (65), but anumber of studies have found associations betweenPCBs and reduced semen quality.In relation to the marked Danish-Finnish differencein incidence of male reproductive disorders describedabove, it is of note that Danish mothers have higherconcentrations of several persistent chemicals in breastmilk compared to Finnish mothers (66,67). In addition toTable 1.Examples of endocrine disrupters and human exposuresources
Endocrine disruptersPolychlorinated biphenyls(PCBs)Phthalates (e.g.diethylphthalate, dibutylphthalate)BrominatedflameretardantsParabens (e.g.butylparaben,propylparaben)Bisphenol-A (e.g.polycarbonate)UV-filters (e.g.3-(4-methylbenzylidene)-camphor, hydroxylatedbenzophenones)Dioxin (e.g.2,3,7,8-tetrachlorodibenzo-p-dioxin)Polyfluorinated chemicals(e.g. PFOA, PFDoA)
Human exposure sourcesTransformers, cutting oils,plastic, paint, foodPaint, plastics, foodwrapping, cosmetics, food,dustBuilding materials,electronic equipment, foodPreservatives in food andcosmeticsBaby and water bottles,electronic equipment, foodSunscreens, colouredindustrial products
By-product fromcombustion processes,foodPaints, impregnation ofclothes and footwear,waxes forfloorsand cars,airFood
Pesticides (e.g.vinclozolin, dieldrin,hexachlorobenzene, DDT/DDE)
Science Policy Briefing 40 – Male Reproductive Health – September 2010|5
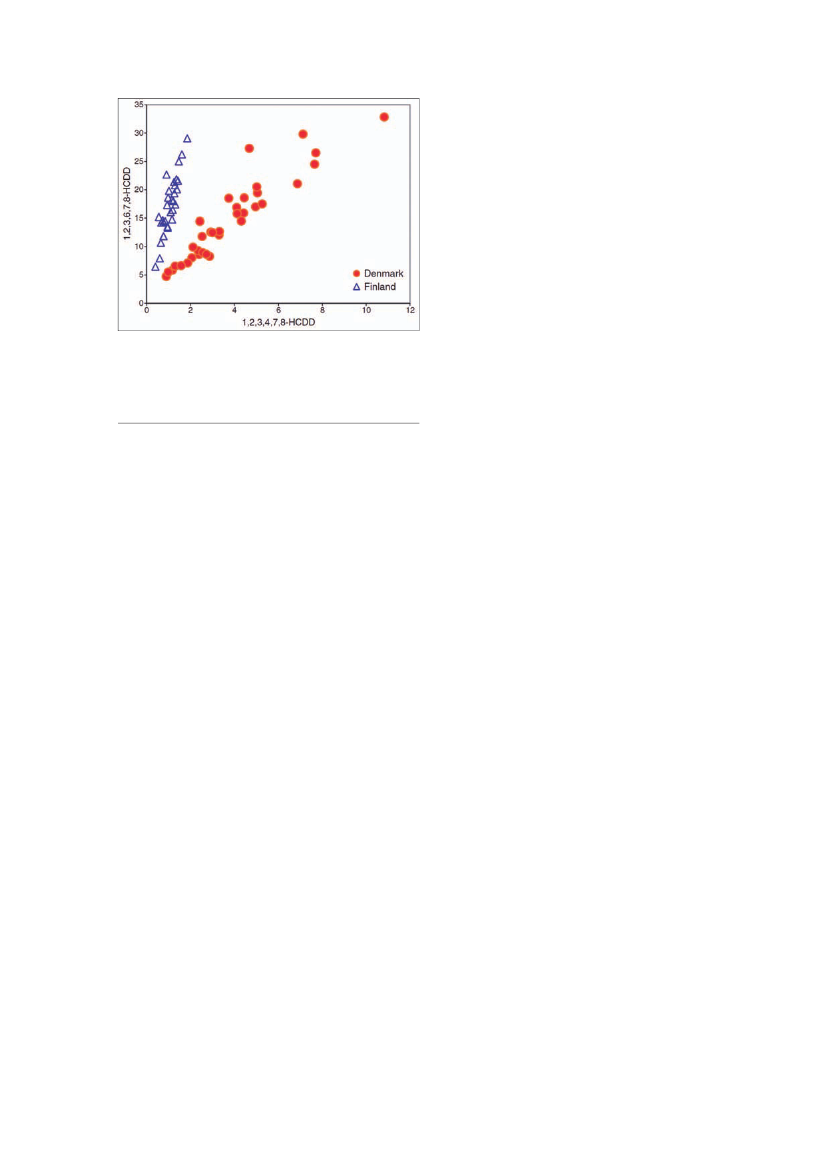
Figure 6.A 2-dimensional scatter plot showing the concentrationof the two chemicals, 1,2,3,4,7,8-HCDD (x-axis) and 1,2,3,6,7,8-HCDD (y-axis), in each breast milk sample (pg/g lipids). The Danish(red) and Finnish (blue) samples are completely separated intotwo distinct groups. In each country, the two chemicals are clearlylinearly correlated. However, the slopes are different in the twogroups (from ref. 67).
have shown that male obesity is associated with reducedsemen quality. Smoking has also been found to impairsemen quality. A meta-analysis published in 1994 basedon 20 studies (75) found that smokers had a significantreduction in sperm concentration and a recent Danishstudy among men from the general population founda dose–response relationship between smoking andsperm motility and total sperm count (76).Interestingly,maternal smoking during pregnancy has a quite pro-nounced negative impact on semen quality among theoffspring indicating that prenatal exposures are alsoimportant (77-80). Maternal smoking in pregnancy hasalso been shown in some (but not all) studies to increasethe risk of hypospadias (81) and cryptorchidism (82,83)in male offspring. On the other hand, a meta-analysishas shown that maternal smoking during pregnancyis not associated with increased risk of TGC in sons(84). Nevertheless, the considerable increase in smok-ing prevalence among young women in most Europeancountries in recent years can only exacerbate the inci-dence of male reproductive problems as some of thesewomen will continue to smoke during pregnancy.
quantitative differences in the exposure levels, the Danishand Finnish children have qualitatively distinct exposurepatterns, typical chemical signatures that exemplify dif-ferences in their environmental impacts (Fig.6) indicatinga higher exposure for Danish infants, and presumablyalso indicating higher exposure during fetal life.Although there is probably enough evidence overall tosupport the conclusion that exposure to EDC, probablyduring fetal life, may have contributed to the increase inmale reproductive health problems, this evidence doesnot provide grounds for concluding that this is the solecausal factor (64). On the other hand, the complexity ofcurrent human exposure to environmental chemicalsand the likelihood for additive effects of similarly actingchemicals, as seen in animal studies, means that identi-fying the importance of the role played by EDC in humanmale reproductive disorders is quite difficult. Despitethis difficulty, there is a strong incentive to improve ourunderstanding in this area, as it is certainly feasible totake steps to minimise exposure to identified causalagents, and this can only have positive effects in termsof improving reproductive health.Epidemiological studies at an international level areurgently needed to provide a definitive association, orits lack, between exposure to individual environmentalchemicals and any of the male reproductive disordersin humans (64). As described above the impact on malereproductive health can be very high.
Genetic factorsThere is growing evidence that genetic and epigeneticfactors play a pivotal role for male reproductive health(85,86,87). The presence of a supernumerary X chro-mosome leads to Klinefelter syndrome (47, XXY), whichis the most frequent chromosomal aneuploidy with anincidence of 1:400 male births and is characterised byhypergonadotropic hypogonadism and infertility. Recentstudies clearly show that methylation and genetic poly-morphisms are impacting the highly variable phenotypeof Klinefelter patients. Moreover, further developmentof microsurgical techniques has led to the recovery ofspermatozoa from these patients, which in principleallows them to father children. However, in more than50% of the patients no sperm can be recovered, indicat-ing that the same chromosomal background could havesignificantly different effects on spermatogenesis.Familial aggregation of TDS disorders indicatesthat genetic factors may be involved in the aetiology.For example, the risk of developing TGC is markedlyincreased among brothers and sons of patients withTGC (88), and likewise cryptorchidism as well as hypo-spadias aggregate among male twin pairs andfirst-,second- and third-degree relatives (89). Besides rarepoint mutations (e.g. SRY mutation) and abnormalchromosome constitutions (e.g. 45X/46, XY), which areassociated with increased risk of TGC, little is knownabout the role that specific genes play in the aetiologyof TDS disorders. Mutations in the AR gene or in thegene encoding the 5-α-reductase type II enzyme, areassociated with cryptorchidism and/or hypospadias, butthese mutations are also extremely rare. Furthermore,there is, to date, virtually no evidence for the existenceof specific genotypes predisposing to adverse effectsof environmental or lifestyle factors (90). Racial differ-ences in TDS, however, indicate a genetic component.US white men exhibit a markedly higher incidence ofTGC than both Afro-American and other non-white USmen (91). Geographical differences in TDS disorders, e.g.
Lifestyle factorsLifestyle factors may also contribute to the observedadverse trends in male reproductive health. During thepast 50 years huge changes in Western lifestyle haveoccurred; for example obesity is reaching epidemicproportions worldwide (68,69) and the prevalence ofsmokers has increased and then more recently declinedin many Western countries (70). Several studies amongmen from the general population or infertile men (71-74)
6|Science Policy Briefing 40 – Male Reproductive Health – September 2010
between Danish and Finnish men as described above,could also reflect genetic differences in susceptibilityto induction of these disorders by EDC and/or lifestylefactors or a combination of both. In this regard, severalScandinavian studies have shown that the incidenceof TGC among Finnishfirstgeneration immigrants toSweden is comparable to the country of origin, whereasamong second generation immigrants it resembles thatof the host country. This strongly suggests that envi-ronmental factors are an essential component in manyTGC cases (92,93). Further research at international levelis needed to get more knowledge about these severereproductive health problems. As with many diseases itseems likely that the risk of developing male reproduc-tive disorders/TDS will involve interplay between genesand the environment.During recent years numerous candidate genesfor male infertility have been screened for mutations.However, it turns out that mutations in autosomal genesare rare and do not play a substantial role in maleinfertility, while in 2% of oligo- or azoospermic men,microdeletions in the male-specific region of the Y chro-mosome can be detected (94,95). Our knowledge aboutX-chromosomal genes and their role in spermatogenesisis scant and should be improved.A new concept has recently been proposed predictingthat single nucleotide polymorphisms (SNPs) either aloneor in combination with other SNPs are associated withmodulation of spermatogenesis. In the worst scenariothese polymorphisms may cause male infertility. Finally,epimutations leading to aberrant methylation of imprintedgenes are considered a clear-cut phenomenon in menwith impaired spermatogenesis. Several studies haveconvincingly shown that sperm morphology and spermcounts are significantly associated with the degree ofnormal methylation patterns of imprinted genes (96).Genetic alterations of the male germline are specifi-cally relevant for patients undergoing ART. It will be ofgreat importance to ensure that the sperm used for ICSIor IVF procedures is as well selected in terms of DNAintegrity as under natural conception. It is biologicallyplausible, and preliminary data indicate, that childrenconceived by ART procedures show an increased riskof developing DNA methylation-specific diseases suchas Beckwith-Wiedemann- or Angelman syndrome (97).Whether these genetic changes are associated with thedisturbed genetic background of infertile couples or withthe IVF procedures remains uncertain at present andhas to be clarified.Thus the researchfieldof epigenetic changes hasgreat importance for male reproductive health andneeds to be more deeply explored as it brings qualitativeaspects of male germ cells into the centre of attentionwhich are highly relevant for the health of offspring con-ceived through ART procedures.
ConclusionsDuring recent years we have witnessed significantadverse trends in reproductive health problems inyoungmen, with large geographical variations. In manyEuropean countries at least 20% of young men exhibitsemen quality below the lower WHO reference level andthis will most likely affect their fertility. The increasinguse of ART also indicates that infertility is a growingproblem. These widespread male reproductive healthproblems may contribute to decreasing birth rates, andthe attendant socio-economic consequences. A signifi-cant proportion of men with TGC, poor semen quality,cryptorchidism and hypospadias may have a TDS ofprenatal origin. The recently observed rapid increasein male reproductive disorders indicates that they arecaused by environmental factors or changes in our life-style rather than genetic factors; this means that suchdisorders are intrinsically preventable, provided that thecause(s) can be identified. Of concern is also the mount-ing evidence that these male reproductive disordersmay be associated with, and may contribute causally to,the explosive increase in cardiovascular and metabolicdiseases in men, possibly via effects on testosteronelevels. The recent recognition of the dynamic interplaybetween testosterone levels and abdominal obesity andits sequelae in men, in combination with the evidencefor a secular decline in testosterone levels in men, sug-gests that the parallel increases in male reproductive andcardiometabolic health disorders may to some extent beinterrelated. Our present understanding of the origin, andespecially of the causes, of human male reproductivedisorders is unfortunately very poor. Increased under-standing would not only improve our ability to preventor treat male reproductive disorders, but would alsohave a much wider impact on aspects of men’s healththat look set to dominate the European scene for thecoming decades. From a socio-economic perspective,the impact of deteriorating male reproductive health inEurope thus looks pervasive.Thus action is needed to improve national and inter-national collaborative research in thefieldof malereproductive health to resolve the many remainingquestions.
Science Policy Briefing 40 – Male Reproductive Health – September 2010|7
Recommendations for a common research strategyin male reproductive healthThere is an urgent need to strengthen and to interlinkresearch in male reproductive health at the national,European and international levels. This should take intoaccount other factors which could interact with reproductivehealth at various levels, such as, for example, the growingobesity-related health issues across Europe or the influenceof EDC. As mentioned above there remain many open ques-tions both at the molecular and at the population/patientlevel so that it is generally important to strengthen transla-tional research to better understand the consequences ofcertain disorders and their underlying mechanisms.Themain recommendationsare therefore the following:•Increase awareness of male reproductive healthissuesCurrently, reproductive health of young men is not consid-ered an important issue (other than sexually transmittedinfections), despite growing evidence that it has a majorinfluence on the frequency of male infertility and subse-quent need for ART. In addition poor male reproductivehealth may be intrinsically linked to general health andlife expectancy. It is therefore important to increaseawareness of the major consequences that can arisefrom reduced male reproductive health.•Strengthen interdisciplinary, translational researchMale reproductive health might be influenced by differ-ent factors. As an example there is growing evidencethat modern lifestyle not only causes obesity, it mayalso adversely affect both sperm counts and bloodtestosterone levels in men. However, the mechanismsinvolved and the long-term health implications arelargely unknown. The susceptibility to develop infertil-ity and reproductive dysfunction/diseases can startduring testicular development as a result of exposureof pregnant women to environmental chemicals.Indeed there is the possibility, shown in animal mod-els, that subferfility may be transmitted through severalgenerations. In light of the current low birth ratesand high need for ART, interdisciplinary, translationalresearch is needed to better understand the differentinteracting factors which can have adverse effects onmale reproductive health.• Implement long-term, epidemiological studiesTo truly understand the etiology of poor male reproductivehealth, it will be critical to mechanistically understandthe genetic and environmental contributions and theirinteractions in male reproductive health. Since environ-ment, as opposed to genetics, can be changed, there isthe possibility to intervene to prevent infertility and otherreproductive diseases as well as co-morbidity factorsby reducing environmental exposures. Therefore it isnecessary to conduct long-term epidemiological studiesto better understand the interacting mechanisms in malereproductive disorders.• Target research effortsBetter understanding of the mechanisms involved in theseprocesses will provide paths forward for improving malereproductive health and will also likely have an impact onwider aspects of general health because of the emerg-ing interconnections between these. It is envisaged thatthe results of such a research effort will be to identifythe means of preventing/minimising occurrence of thedisorders rather than the lengthy and costly developmentof drug treatments.
Proposed funding instruments• Strengthen national fundingTransdisciplinary, translational national research networksin human male reproductive health and fertility/infertil-ity should be established as a focus area by nationalresearch councils and should be part of and contributeto the European network described below.National funds needed will vary with size of country,probably between 1 and 5million euros per country peryear.• Establish a European transdisciplinary,translational ‘Research Network of Excellence’in male reproductive healthThe role of such a network would be to evaluate thecauses and consequences of the current low Europeanfertility rates. The network should include expertise inandrology, endocrinology, management of infertility (IVF,ICSI), EDC, environmental health sciences, experimen-tal systems, demography, sociology, epidemiology andbioinformatics/statistics. This network should use thismultidisciplinary expertise toestablish robust meth-odsfor accurately determining the extent of involuntaryinfertility across Europe, especially male-mediated infer-tility, and theimportance of societal factorsincludingexposures to environmental chemicals(individuallyand in mixtures), andgenetic background.It shouldutilise available methods, birth and adult cohorts,to tease apart the relative importance of developmentalversus adult causes of low sperm counts/infertility; thisshould take into account and make use of establishedgeographical differences in sperm counts/related malereproductive disorders within Europe. The network shouldmaintain quality control schemes to establish high andconsistent standards of analytical methodology andpatient care and include ascientific advisory boardto assess progress and integration. Suggested fundinglevel:5millioneuros per year for 10 years.• Establish links between the proposed Europeanresearch network and similar networks in the US,Asia and other parts of the worldSuch transnational cooperation would enable coordi-nation of research, intervention and prevention effortsacross the globe. The links should result in the forma-tion of an effective international taskforce to tackle thealarmingly low fertility rates and other male reproduc-tive diseases/dysfunctions in industrialised countriesacross the world, including all European countries, Japan,South Korea, Singapore as well as the US and develop-ing countries. It is expected that the international groupswill depend on their own core funding. However, runningthe taskforce activities (workshops, exchange of youngscientists, common publications) are estimated to cost1millioneuros per year (European share: 25%).
8|Science Policy Briefing 40 – Male Reproductive Health – September 2010
References(1) Lutz W. Fertility rates and future population trends: willEurope’s birth rate recover or continue to decline?Int J Androl2006 February;29(1):25-33.(2) United Nations. World Population Prospects: The 2006Revision. New York, United Nations 2007.(3) Andersen AN, Carlsen E, Loft A. Trends in the use ofintracytoplasmatic sperm injection marked variabilitybetween countries.Hum Reprod Update2008November-December;14(6):593-604.(4) Nyboe AA, Erb K. Register data on AssistedReproductive Technology (ART) in Europe including adetailed description of ART in Denmark.Int J Androl2006 February;29(1):12-6.(5) Skakkebæk NE, Rajpert-De Meyts E, Main KM.Testicular dysgenesis syndrome: an increasinglycommon developmental disorder with environmentalaspects.Hum Reprod2001 May;16(5):972-8.(6) Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB.Inverse association of testosterone and the metabolicsyndrome in men is consistent across race and ethnicgroups.J Clin EndocrinolMetab 2008 September;93(9):3403-10.(7) Laughlin GA, Barrett-Connor E, Bergstrom J. Lowserum testosterone and mortality in older men.J ClinEndocrinol Metab2008 January;93(1):68-75.(8) Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TWet al.Hypothalamic-pituitary-testicular axis disruptionsin older men are differentially linked to age andmodifiable risk factors: the European Male Aging Study.J Clin Endocrinol Metab2008 July;93(7):2737-45.(9) Rodriguez A, Muller DC, Metter EJ, Maggio M, HarmanSM, Blackman MRet al.Aging, androgens, and themetabolic syndrome in a longitudinal study of aging.JClin EndocrinolMetab 2007 September;92(9):3568-72.(10) Feldman HA, Longcope C, Derby CA, Johannes CB,Araujo AB, Coviella ADet al.Age trends in the level ofserum testosterone and other hormones in middle-agedman: longitudinal results from the Massachusetts MaleAging Study.J Clin Endocrinol Metab2002;87:589-98.(11) Mulligan T, Frick MF, Zuraw QC, Stemhagen A,McWhirter C. Prevalence of hypogonadism in malesaged at least 45 years: the HIM study.Int J Clin Pract2006 July;60(7):762-9.(12) Travison TG, Araujo AB, O’donnell AB, Kupelian V,McKinlay JB. A population-level decline in serumtestosterone levels in American men.J Clin EndocrinolMetab2007 January;92(1):196-202.(13) Andersson AM, Jensen TK, Juul A, Petersen JH,Jørgensen T, Skakkebæk NE. Secular decline in maletestosterone and sex hormone binding globulin serumlevels in Danish population surveys.J Clin EndocrinolMetab2007;92:4696-705.(14) Nielsen TL, Hagen C, Wraae K, Brixen K, PetersenPH, Haug Eet al.Visceral and subcutaneous adiposetissue assessed by magnetic resonance imaging inrelation to circulating androgens, sex hormone-bindingglobulin, and luteinizing hormone in young men.J ClinEndocrinol Metab2007 July;92(7):2696-705.(15) Carlsen E, Giwercman A, Keiding N, Skakkebæk NE.Evidence for decreasing quality of semen during past50 years.BMJ1992;305:609-13.(16) Swan SH, Elkin EP, Fenster L. The question of decliningsperm density revisited: an analysis of 101 studiespublished 1934-1996.Environ Health Perspect2000;108:961-6.(17) Jouannet P, Wang C, Eustache F, Jensen TK, AugerJ. Semen quality and male reproductive health: thecontroversy about human sperm concentration decline.APMIS2001;109:333-44.
(18) Andersen AG, Jensen TK, Carlsen E, Jørgensen N,Andersson A-M, Krarup Tet al.High frequency of sub-optimal semen quality in an unselected population ofyoung men.Hum Reprod2000;15:366-72.(19) Jørgensen N, Carlsen E, Nermoen I, Punab M,Suominen J, Andersen AGet al.East-West gradientin semen quality in the Nordic-Baltic area: a study ofmen from the general population in Denmark, Norway,Estonia and Finland.Hum Reprod2002 August;17(8):2199-208.(20) Bonde JP, Ernst E, Jensen TK, Hjollund NH, KolstadH, Henriksen TBet al.Relation between semenquality and fertility: a population-based study of 430first-pregnancyplanners.Lancet1998 October 10;352(9135):1172-7.(21) Jensen TK, Jacobsen R, Christensen K, Nielsen NC,Bostofte E. Good semen quality and life expectancy:a cohort study of 43,277 men.Am J Epidemiol2009September 1;170(5):559-65.(22) Jørgensen N, Andersen A-G, Eustache F, Irvine DS,Suominen J, Petersen JHet al.Regional differences insemen quality in Europe.Hum Reprod2001;16:1012-9.(23) Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, HatchMet al.Geographic differences in semen quality offertile US males.Environ Health Perspect2003 April;111:414-20.(24) Iwamoto T, Hoshino T, Nishida T, Baaba K, Matsusita T,Kaneko Set al.Semen quality of 324 fertile Japanesemen.Hum Reprod2006;21:760-5.(25) Chia SE, Tay SK, Lim ST. What constitutes a normalseminal analysis? Semen parameters of 243 fertilemen.Hum Reprod1998 December;13(12):3394-8.(26) Jacobsen R, Bostofte E, Engholm G, Hansen J, OlsenJH, Skakkebæk NEet al.Risk of testicular cancer inmen with abnormal semen characteristics: cohortstudy.BMJ2000 September 30;321(7264):789-92.(27) Møller H, Skakkebæk NE. Risk of testicular cancer insubfertile men: case-control study.BMJ1999 February27;318(7183):559-62.(28) Huyghe E, Matsuda T, Thonneau P. Increasingincidence of testicular cancer worldwide: a review.JUrol2003 July;170(1):5-11.(29) Huyghe E, Plante P, Thonneau PF. Testicular cancervariations in time and space in Europe.Eur Urol2007March;51(3):621-8.(30) Richiardi L, Bellocco R, Adami HO, Torrang A, BarlowL, Hakulinen T et al. Testicular cancer incidence ineight northern European countries: secular and recenttrends.Cancer Epidemiol Biomarkers Prev2004December;13:2157-66.(31) Skakkebæk NE, Berthelsen JG, Giwercman A, MullerJ. Carcinoma-in-situ of the testis: possible origin fromgonocytes and precursor of all types of germ celltumours except spermatocytoma.Int J Androl1987February;10(1):19-28.(32) Rajpert-De Meyts E. Developmental model for thepathogenesis of testicular carcinomain situ:geneticand environmental aspects.Hum Reprod Update2006May;12(3):303-23.(33) Jørgensen N, Asklund C, Carlsen E, Skakkebæk NE.Coordinated European investigations of semen quality:results from studies of Scandinavian young men isa matter of concern.Int J Androl2006 February;29(1):54-61.(34) Akre O, Lipworth L, Cnattingius S, Sparen P, EkbomA. Risk factor patterns for cryptorchidism andhypospadias.Epidemiology1999 July;10(4):364-9.(35) Weidner IS, Møller H, Jensen TK, Skakkebæk NE. Riskfactors for cryptorchidism and hypospadias.J Urol1999 May;161(5):1606-9.
Science Policy Briefing 40 – Male Reproductive Health – September 2010|9
(36) Lee PA, Coughlin MT. Fertility after bilateralcryptorchidism. Evaluation by paternity, hormone, andsemen data.Horm Res2001;55(1):28-32.(37) Asklund C, Jensen TK, Main KM, Sobotka T,Skakkebæk NE, Jorgensen N. Semen quality,reproductive hormones and fertility of men operated forhypospadias.Int J Androl2009 March 5;33(1)80-7.(38) Prener A, Engholm G, Jensen OM. Genital anomaliesand risk for testicular cancer in Danish men.Epidemiology1996 January;7(1):14-9.(39) Toppari J, Kaleva M, Virtanen HE. Trends in theincidence of cryptorchidism and hypospadias, andmethodological limitations of registry-based data.Hum Reprod Update2001 May;7(3):282-6.(40) Boisen KA, Kaleva M, Main KM, Virtanen HE, HaavistoA-M, Schmidt IMet al.Difference in prevalence ofcongenital cryptorchidism in infants between twoNordic countries.Lancet2004;363:1264-9.(41) Acerini CL, Miles HL, Dunger DB, Ong KK, HughesIA. The descriptive epidemiology of congenital andacquired cryptorchidism in a UK infant cohort.Arch DisChild2009 June 18;94:868-72.(42) Boisen KA, Chellakooty M, Schmidt IM, Kai CM,Damgaard IN, Suomi AMet al.Hypospadias in acohort of 1072 Danish newborn boys: Prevalence andrelationship to placental weight, anthropometricalmeasurements at birth, and reproductive hormonelevels at 3 months of age.J Clin Endocrinol Metab2005 May 3;90:4041-6.(43) Jensen TK, Sobotka T, Hansen MA, Pedersen AT, LutzW, Skakkebæk NE. Declining trends in conceptionrates in recent birth cohorts of native Danish women: apossible role of deteriorating male reproductive health.Int J Androl2008;31:81-92.(44) Samlede danske ART behandlingsresultater [TotalDanish Treatment Results with ART]. Availablefrom: http://www.fertilitetsselskab.dk/index.php?option=com_content&view=article&id=68&Itemid=82 (accessed Sept-22, 2010)(45) Andersson AM, Jørgensen N, Larsen LF, Rajpert-DeMeyts E, Skakkebæk NE. Impaired Leydig cell functionin infertile men: a study of 357 idiopathic infertile menand 318 proven fertile controls.J Clin Endocrinol Metab2004;89:3161-7.(46) De Kretser DM. Editorial: Is spermatogenic damageassociated with Leydig cell dysfunction?J ClinEndocrinol Metab2004 July;89(7):3158-60.(47) Jensen TK, Andersson A-M, Jørgensen N, AndersenA-G, Carlsen E, Petersen JHet al.Body mass index inrelation to semen quality and reproductive hormonesamong 1,558 Danish men.Fertil Steril2004;82:863-70.(48) Welsh M, Saunders PT, Fisken M, Scott HM,Hutchison GR, Smith LBet al.Identification inrats of a programming window for reproductivetract masculinization, disruption of which leads tohypospadias and cryptorchidism.J Clin Invest2008April;118(4):1479-90.(49) Toppari J. Environmental endocrine disrupters.SexDev2008;2(4-5):260-7.(50) Fisher JS, Macpherson S, Marchetti N, Sharpe RM.Human ‘testicular dysgenesis syndrome’: a possiblemodel usingin-uteroexposure of the rat to dibutylphthalate.Hum Reprod2003 July;18(7):1383-94.(51) Edwards TM, Moore BC, Guillette LJ, Jr. Reproductivedysgenesis in wildlife: a comparative view.Int J Androl2006 February;29(1):109-21.(52) Kelce WR, Wilson EM. Environmental antiandrogens:developmental effects, molecular mechanisms, andclinical implications.J Mol Med1997 March;75(3):198-207.
(53) Dodds E, Lawson W. Synthetic oestrogenic agentswithout the phenantrene nucleus.Nature1936;137:996.(54) Welshons WV, Nagel SC, vom Saal FS. Large effectsfrom small exposures. III. Endocrine mechanismsmediating effects of bisphenol A at levels of humanexposure.Endocrinology2006 June;147(6Suppl):S56-S69.(55) Vandenberg LN, Hauser R, Marcus M, Olea N,Welshons WV. Human exposure to bisphenol A (BPA).Reprod Toxicol2007 August;24(2):139-77.(56) Vinggaard AM, Hass U, Dalgaard M, Andersen HR,Bonefeld-Jorgensen E, Christiansen Set al.Prochloraz:an imidazole fungicide with multiple mechanisms ofaction.Int J Androl2006 February;29(1):186-92.(57) Christiansen S, Scholze M, Axelstad M, Boberg J,Kortenkamp A, Hass U. Combined exposure to anti-androgens causes markedly increased frequenciesof hypospadias in the rat.Int J Androl2008 April;31(2):241-8.(58) Sharpe RM. “Additional” effects of phthalate mixtureson fetal testosterone production.Toxicol Sci2008September;105(1):1-4.(59) Lopez-Espinosa MJ, Granada A, Carreno J, SalvatierraM, Olea-Serrano F, Olea N. Organochlorine pesticidesin placentas from Southern Spain and some relatedfactors.Placenta2007 July;28(7):631-8.(60) Kortenkamp A. Low dose mixture effects of endocrinedisrupters: implications for risk assessment andepidemiology.Int J Androl2008 April;31(2):233-40.(61) Damgaard IN, Skakkebæk NE, Toppari J, Virtanen HE,Shen H, Schramm KWet al.Persistent pesticides inhuman breast milk and cryptorchidism.Environ HealthPerspect2006 July;114(7):1133-8.(62) Main KM, Kiviranta H, Virtanen HE, Sundqvist E,Tuomisto JT, Tuomisto Jet al.Flame retardantsin placenta and breast milk and cryptorchidism innewborn boys.Environ Health Perspect2007 October;115(10):1519-26.(63) Andersen HR, Schmidt IM, Grandjean P, Jensen TK,Budtz-Jorgensen E, Kjaerstad MBet al.ImpairedReproductive Development in Sons of WomenOccupationally Exposed to Pesticides duringPregnancy.Environ Health Perspect2008 April;116(4):566-72.(64) Sharpe RM. Male reproductive health disorders and thepotential role of exposure to environmental chemicals.ChemTrust2009.(65) Hauser R. The environment and male fertility: recentresearch on emerging chemicals and semen quality.Semin Reprod Med2006 July;24(3):156-67.(66) Shen H, Main KM, Andersson AM, Damgaard IN,Virtanen HE, Skakkebæk NEet al.Concentrations ofpersistent organochlorine compounds in human milkand placenta are higher in Denmark than in Finland.Hum Reprod2008 January;23(1):201-10.(67) Krysiak-Baltyn K, Toppari J, Skakkebæk NE, JensenTS, Virtanen HE, Schramm KWet al.Country-specificchemical signatures of persistent environmentalcompounds in breast milk.Int J AndrolSeptember2009;33(2):270-8.(68) Caballero B. The global epidemic of obesity: anoverview.Epidemiol Rev2007;29:1-5.(69) Mokdad AH, Bowman BA, Ford ES, Vinicor F, MarksJS, Koplan JP. The continuing epidemics of obesity anddiabetes in the United States.JAMA2001 September12;286(10):1195-200.(70) Molarius A, Parsons RW, Dobson AJ, Evans A,Fortmann SP, Jamrozik Ket al.Trends in cigarettesmoking in 36 populations from the early 1980s to themid-1990s:findingsfrom the WHO MONICA Project.Am J Public Health2001 February;91(2):206-12.
10|Science Policy Briefing 40 – Male Reproductive Health – September 2010
(71) Hammoud AO, Wilde N, Gibson M, Parks A, CarrellDT, Meikle AW. Male obesity and alteration in spermparameters.Fertil Steril2008 December;90(6):2222-5.(72) Kort HI, Massey JB, Elsner CW, Mitchell-Leef D,Shapiro DB, Witt MAet al.Impact of body mass indexvalues on sperm quantity and quality.J Androl2006May;27(3):450-2.(73) Magnusdottir EV, Thorsteinsson T, ThorsteinsdottirS, Heimisdottir M, Olafsdottir K. Persistentorganochlorines, sedentary occupation, obesity andhuman male subfertility.Hum Reprod2005 January;20(1):208-15.(74) Koloszar S, Fejes I, Zavaczki Z, Daru J, Szollosi J,Pal A. Effect of body weight on sperm concentrationin normozoospermic males.Arch Androl2005 July;51(4):299-304.(75) Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarettesmoking and sperm density: a meta-analysis.FertilSteril1994 January;61(1):35-43.(76) Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS,Jensen MS, Toft G, Bonde JP. Is smoking a risk factorfor decreased semen quality? A cross-sectionalanalysis.Hum Reprod2007 January;22(1):188-96.(77) Storgaard L, Bonde JP, Ernst E, Spano M, AndersenCY, Frydenberg Met al.Does smoking duringpregnancy affect sons’ sperm counts?Epidemiology2003 May;14(3):278-86.(78) Ramlau-Hansen CH, Thulstrup AM, Storgaard L, ToftG, Olsen J, Bonde JP. Is prenatal exposure to tobaccosmoking a cause of poor semen quality? A follow-upstudy.Am J Epidemiol2007 June 15;165(12):1372-9.(79) Jensen TK, Jorgensen N, Punab M, Haugen TB,Suominen J, Zilaitiene Bet al.Association ofin uteroexposure to maternal smoking with reduced semenquality and testis size in adulthood: a cross-sectionalstudy of 1,770 young men from the general populationinfiveEuropean countries.Am J Epidemiol2004January 1;159(1):49-58.(80) Ratcliffe JM, Gladen BC, Wilcox AJ, Herbst AL. Doesearly exposure to maternal smoking affect future fertilityin adult males?Reprod Toxicol1992;6(4):297-307.(81) Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, deGier RP, Roeleveld N. Risk factors for hypospadias.EurJ Pediatr2007 July;166:671-8.(82) Thorup J, Cortes D, Petersen BL. The incidence ofbilateral cryptorchidism is increased and the fertilitypotential is reduced in sons born to mothers whohave smoked during pregnancy.J Urol2006 August;176(2):734-7.(83) Jensen MS, Toft G, Thulstrup AM, Bonde JP, OlsenJ. Cryptorchidism according to maternal gestationalsmoking.Epidemiology2007;18:197-8.(84) Tuomisto J, Holl K, Rantakokko P, Koskela P, HallmansG, Wadell Get al.Maternal smoking during pregnancyand testicular cancer in the sons: A nested case-controlstudy and a meta-analysis.Eur J Cancer2009 February21;45(9):1640-8.(85) Tüttelmann F, Rajpert-De Meyts E, Nieschlag E, SimoniM. Gene polymorphisms and male infertility - a meta-analysis and literature review.RBMOnline2007;15:643-58.(86) Giwercman A, Kledal T, Schwartz M, Giwercman YL,Leffers H, Zazzi Het al.Preserved male fertility despitedecreased androgen sensitivity caused by a mutationin the ligand-binding domain of the androgen receptorgene.J Clin Endocrinol Metab2000 June;85(6):2253-9.(87) McElreavey K, Quintana-Murci L. Y chromosomehaplogroups: a correlation with testicular dysgenesissyndrome?APMIS2003 January;111(1):106-13.(88) Lutke Holzik MF, Rapley EA, Hoekstra HJ, SleijferDT, Nolte IM, Sijmons RH. Genetic predisposition to
testicular germ-cell tumours.Lancet Oncol2004 June;5(6):363-71.(89) Schnack TH, Zdravkovic S, Myrup C, WestergaardT, Wohlfahrt J, Melbye M. Familial aggregation ofcryptorchidism – a nationwide cohort study.Am JEpidemiol2008 June 15;167(12):1453-7.(90) Giwercman A, Rylander L, Hagmar L, Giwercman YL.Ethnic differences in occurrence of TDS - genetics and/or environment?Int J Androl2006 February;29(1):291–7.(91) Shah MN, Devesa SS, Zhu K, McGlynn KA. Trendsin testicular germ cell tumours by ethnic group in theUnited States.Int J Androl2007 August;30(4):206-13.(92) Hemminki K, Li X. Cancer risks in Nordic immigrantsand their offspring in Sweden.Eur J Cancer2002December;38(18):2428-34.(93) Myrup C, Westergaard T, Schnack T, Oudin A, RitzC, Wohlfahrt Jet al.Testicular cancer risk infirst-andsecond-generation immigrants to Denmark.J NatlCancer Inst2008 January 2;100(1):41-7.(94) Simoni M, Tuttelmann F, Gromoll J, Nieschlag E.Clinical consequences of microdeletions of the Ychromosome: the extended Munster experience.Reprod Biomed Online2008 February;16(2):289-303.(95) Krausz C, Giachini C, Xue Y, O’Bryan MK, Gromoll J,Rajpert-De Meyts Eet al.Phenotypic variation withinEuropean carriers of the Y-chromosomal gr/gr deletionis independent of Y-chromosomal background.J MedGenet2009 January;46(1):21-31.(96) Poplinski A, Tuttelmann F, Kanber D, HorsthemkeB, Gromoll J. Idiopathic male infertility is stronglyassociated with aberrant methylation of MEST andIGF2/H19 ICR1.Int J Androl2009 October;33(4)642-9.(97) Manipalviratn S, DeCherney A, Segars J. Imprintingdisorders and assisted reproductive technology.FertilSteril2009 February;91(2):305-15.
AbbreviationsART:assisted reproduction techniquesEDC:endocrine disrupting chemicalsICSI:intracytoplasmic sperm injectionIVF:in vitrofertilisationTDS:testicular dysgenesis syndromeTGC:testicular germ cell cancerTNCR:total natural conception rate
DefinitionsTesticular Germ Cell Cancer• Commonest cancer in young men• Associated with impaired semen quality and lowerfertility rates• Aetiology unknownCongenital Malformations• Cryptorchidism: undescended testis• Hypospadias: incomplete fusion of the urethral foldsTotal Natural Conception Rate• Includes total number of births and induced abortions• Excludes births after the use of ARTEndocrine disrupting chemicals (EDC)Definition by WHO, International Programmeon Chemical Safety (IPCS):Exogenous substances that alter function(s) of theendocrine system and consequently cause adversehealth effects in an intact organism, or its progeny,or (sub)populations
Science Policy Briefing 40 – Male Reproductive Health – September 2010|11
Expert GroupThis ESF Science Policy Briefing has been writtenby the following Experts:• Professor Nicolas Olea,University of Granada, Hospital UniversityS. Cecilio-CIBERESP, Granada, Spain•Professor Richard Sharpe,MRC Human Reproductive Sciences Unit,Queen’s Medical Research Institute, Edinburgh,UnitedKingdom•Professor Bernard Jegou,GERHM-INSERM U625, Université de Rennes I,Rennes, France•Professor Jorma Toppari,University of Turku, Departments of Physiologyand Pediatrics, Turku, Finland•Professor Niels E. Skakkebæk(corresponding author),Rigshospitalet, University Department of Growthand Reproduction, Copenhagen, Denmark•Professor Dr Stefan Schlatt,University Hospital of Münster, Centre ofReproductive Medicine and Andrology, Münster,GermanyUS participant in the group:•Dr Jerry Heindel,NIEHS, Division of Extramural Research andTraining, Research Triangle Park, USA
AcknowledgementParts of this report have previously been presented byNiels Skakkebæk at a WHO workshop in Tokyo, 2008,and these parts will be included in the proceedingsfromthat meeting in a modified form.
This ESF Science Policy Briefing has beenprepared under the responsibility of the StandingCommittee of the European Medical ResearchCouncils (EMRC)•Professor Liselotte Højgaard,EMRC Chair, Clinic of Clinical Physiology, NuclearMedicine and PET, University of Copenhagen andTechnical University, Copenhagen, Denmark•Dr Stephane Berghmans,Head of Unit, Medical Sciences•Dr Maria Manuela Nogueira,Science Officer, Medical Sciences Unit•Dr Kirsten Steinhausen,Science Officer, Medical Sciences Unit
The European Science Foundation (ESF) was established in 1974 to provide a common platform for its MemberOrganisations to advance European research collaboration and explore new directions for research. It is an inde-pendent organisation, owned by 79 Member Organisations, which are research funding organisations and researchperforming organisations, academies and learned societies from 30 countries. ESF promotes collaboration in researchitself, in funding of research and in science policy activities at the European level.Print run: 1 000 – September 2010
ISBN: 978-2-918428-23-7
1 quai Lezay-Marnésia | BP 9001567080 Strasbourg cedex | FranceTel: +33 (0)3 88 76 71 00 | Fax: +33 (0)3 88 37 05 32www.esf.org