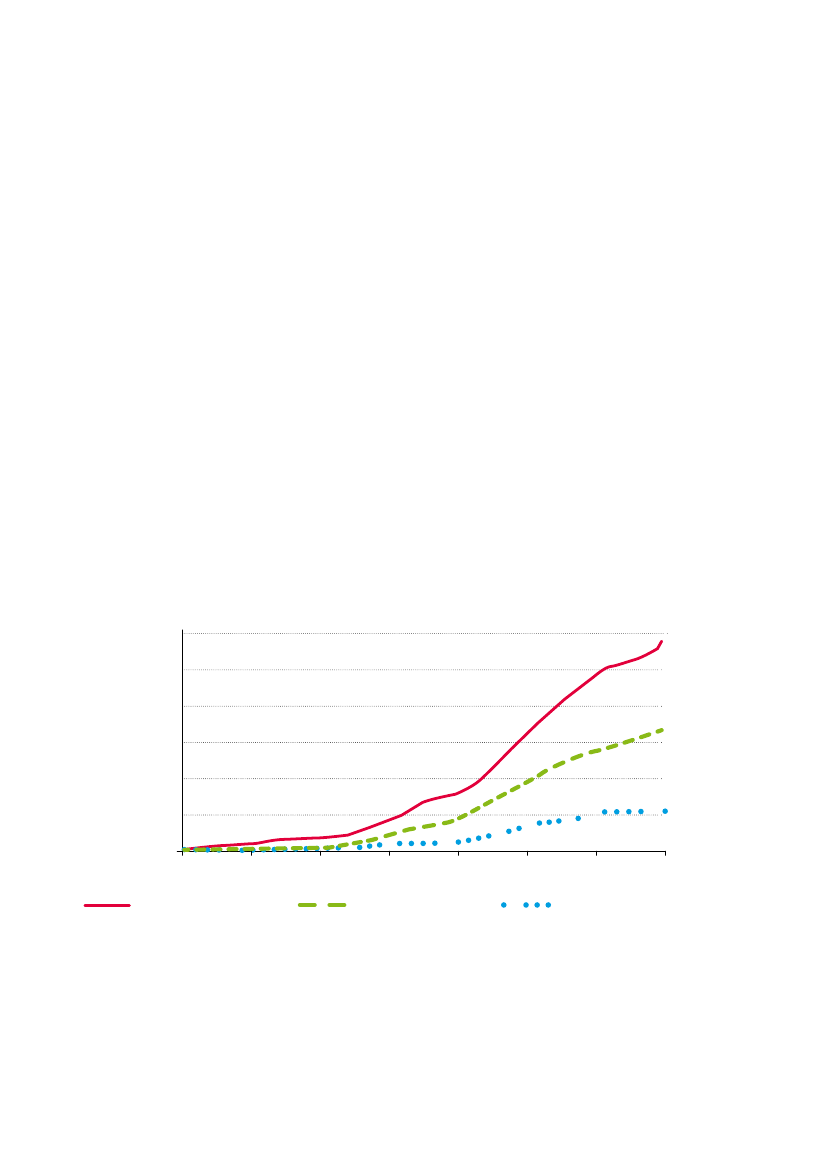Miljø- og Planlægningsudvalget 2010-11 (1. samling)
MPU Alm.del Bilag 743
Offentligt
Herbicide toleranceand GM cropsWhy the world should beReady to Round Up glyphosateEXECUTIVESUMMARY& REPORTJune 2011
Canada, 2007: GreenpeaceHerbicide tolerance and GM cropsactivists created a 60Why the world should bemetre-wide question markin a cropfield containingReady to Round Up glyphosateMonsanto’s geneticallyengineered corn.
ContentsHerbicide tolerance and GM cropsThis report examines the environmental and health implications of the widespread and intensiveuse of the herbicide glyphosate in association with GM (Roundup Ready) crops.
Executive Summary1) Introduction1.1 Glyphosate: how does it work?1.2 GM crops: a perfect match for Roundup1.3 Other uses of glyphosate1.4 Summary
499101010
2) Glyphosate impacts on human health2.1 Chronic effects2.2 Acute effects2.3 Summary
13131616
3) Glyphosate residues in foodSummary
1920
4) Glyphosate in waterConclusion
2324
5) Glyphosate impacts on biodiversity5.1 Direct toxic effects5.2 Does glyphosate affect the nervous system?5.3 Impacts on non-target plants5.4 Summary
2626272828
6) Glyphosate impacts on the soil-plant system6.1 Availability of glyphosate in the soil6.2 Activity and abundance of soil microbes6.3 Reduced nutrient uptake by plants6.4 Increased vulnerability to plant diseases6.5 Case studies of glyphosate impacts on soil-plant system6.6 Summary
29292930303232
7) Weed resistance and glyphosate - the failure of GM Roundup Ready technology7.1 A false dawn7.2 Monsanto’s Reaction to reports of resistant weeds7.3 False solutions create more problems7.4 Conclusion
3333343636
8) ConclusionsReferences
3839
2GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
For more information contact:enquiries@greenpeace.orgenquiry@gmfreeze.org
AuthorsPete Riley,GM FreezeDr. Janet Cotter,GreenpeaceResearch Laboratories, University ofExeter, UKMarco Contiero,GreenpeaceEuropean UnitDr Meriel Watts,Pesticides ActionNetwork Asia Pacific (Chapter 2)Edited byBecky PriceMyrto Pispini,Greenpeace InternationalDesigned byAtomo DesignJN 363Greenpeace Research laboratoriesTechnical Note 03/2011GRL-TN 03/2011Published byGreenpeace InternationalOttho Heldringstraat 51066 AZ AmsterdamThe Netherlandsgreenpeace.orgGreenpeace Research LaboratoriesInnovation Centre 2, Rennes DriveUniversity of Exeter,UK EX4 4RNGM FreezeRegistered Office50 South Yorkshire BuildingsSilkstone Common, BarnsleyUK S75 4RJgmfreeze.org
� GREENPEACE / MICHAEL DESJARDINS
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 20113
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Executive SummaryGlyphosate is the active ingredient in manyherbicides sold throughout the world, includingthe well-known formulation, Roundup. Glyphosate-based herbicides are used widely for weed controlbecause they are non-selective; glyphosate killsall vegetation.Glyphosate has been promoted as ‘safe’. However,mounting scientific evidence questions the safetyof glyphosate and its most well known formulation,Roundup.The evidence detailed in this reportdemonstrates that glyphosate-based productscan have adverse impacts on human and animalhealth, and that a review of their safety forhuman and animal health is urgently needed.The widespread and increasingly intensive use of glyphosate inassociation with the use of GM (genetically modified, also calledgenetically engineered or GE) crops poses further risks to theenvironment and human health. GM crops specifically engineered tobe tolerant to glyphosate are known as ‘Roundup Ready’ (RR). TheseRR varieties allow farmers to spray the herbicide over the top of thegrowing crop, killing virtually all weeds without affecting the crop.The use of glyphosate on GM RR crops such as soy, maize and cottonhas increased dramatically in North and South America, where they arepredominantly grown.GM RR crops are marketed by the US agrochemical giant Monsanto,and are associated with its own formulation of glyphosate herbicide,Roundup. Monsanto’s sales pitch to farmers promised, and still does,reduced labour and financial savings by simplifying and reducing thecosts of weed control. The reality is turning out to be different, withincreasing health, biodiversity and environmental concerns and thedevelopment of weed resistance.Given the problems that are now evident, no new GMglyphosate-tolerant crops should be authorised. In broaderterms, GM herbicide-tolerant crops have been developed for anindustrial farming model. They are therefore intrinsically linkedto unsustainable farming practices that damage the basicnatural resources food production is based upon, and theircultivation should be banned.
4GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Exposure to glyphosatePeople, plants and animals can be exposed to glyphosate andRoundup in many ways. Farmers, bystanders and other operators canbe exposed during its application, and neighbouring natural habitatsby drift from the area where it is being applied. Aerial application isused on some crops, such as on the vast monoculture plantations ofGM RR soya in the Americas, which greatly increases the chances ofaccidental exposure of neighbouring populations or habitats.Exposure to glyphosate and Roundup also occurs via their residues,frequently found in food and the environment. The Maximum ResidueLevels (MRLs) in food for glyphosate and its breakdown product wereagreed by the UN-based Codex Alimentarius Commission in 2006,but appear to be related more to the type of agricultural practicescharacteristic of each food crop rather than to safety thresholds forhuman health.In light of the new scientific evidence on the health andenvironmental impacts of glyphosate it is essential tore-evaluate MRLs in order to align them with updated safetyassessments.In the environment, glyphosate can held in the soil by binding toparticles but, depending on soil chemistry, can also leach intogroundwater. Glyphosate can also wash directly into drains andsurface waters and it has been detected in both. Glyphosate andits degradation product have been detected in studies of drainagesurface waters in Canada, the US and Denmark. These finding haveimplications for surface water quality and drinking water quality.Given the evidence that glyphosate can cause harm to health and theenvironment, the leaching of glyphosate has also serious implicationsfor aquatic life.Glyphosate is present in soils, waters and our food as a resultof its use as an herbicide. Therefore, rigorous assessment ofthe safety of glyphosate to plant, humans and animals is ofgreat importance.
Human health problems related to glyphosateIndependent scientific studies are underscoring the call for an urgentreassessment of glyphosate and its related products. These studiesassociate exposure to glyphosate with a number of negative effects onhuman and animal health, including long term or chronic effects:■■
Birth defects in the Argentinean state of Chaco,where GMsoya and rice crops are heavily sprayed with glyphosate, increasednearly fourfold over the years 2000 to 2009. Similar defects werealso found in woman from Paraguay exposed to glyphosate-basedherbicides during pregnancy. These defects were compatiblewith those induced in laboratory experiments at much lowerconcentrations than normal commercial glyphosate concentrations.Glyphosate is a suspected endocrine disruptor.This meansit could disrupt production of vital reproductive hormones, suchas progesterone and oestrogen. Published studies demonstratevarious endocrine effects in animals and human cells associatedwith glyphosate.Studies of illness patterns human populations (epidemiologicalstudies) have linked glyphosate exposure tonon-Hodgkin’slymphoma(a type of blood cancer) whilst laboratory studies haveconfirmed that glyphosate and/or its associated products exhibitcharacteristics typical of cancer causing agents (i.e. genotoxicityor mutagenicity) in animals and both human and animal. Together,these studies suggest that glyphosate may contribute to cancer.Evidence that glyphosatemay also affect the nervous systemand may even be implicated in Parkinson’s disease.
■■
■■
Scientific evidence highlighting these health effects must betaken very seriously. An urgent reassessment of the healthimpacts of glyphosate and its related products must take place.
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 20115
� GREENPEACE / GUSTAVO GILABERT
Glyphosate ispresent in soils,waters and ourfood as a result ofits widespread usewith GM RoundupReady crops.
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Executive Summary (cont.)Glyphosate affects biodiversityGlyphosate can impact on biodiversity in a number of different waysand can have short and long term, as well as direct and indirectnegative effects. Evidence is accumulating that glyphosate can havea damaging impact on aquatic organisms as a result of its normaluse in agriculture or forestry. Several studies have suggested that,under close-to-field conditions, glyphosate-based products, includingRoundup, have a direct toxic effect on the adults and tadpoles of arange of amphibian species. Despite these findings, Monsanto stillclaims that Roundup has ‘noadverse effect on aquatic animals’(Monsanto 2010a).Many aquatic animals - from microscopic algae to fish and mussels- have been found to be affected by exposure to glyphosate and/or Roundup. The observed effects included: shorter life spansand reduced reproductive rates in rotifers (a type of freshwaterinvertebrate); changes in population structure in phyto- (or plant-)plankton; increased mortality in aquatic worms; and changes in livercells in carp. A recent study found genotoxic effects in the red bloodcells of European eels when exposed to Roundup for a short period.There is also a suggestion that glyphosate may affect the nervoussystem of aquatic animals in a manner similar to an organophosphate.Glyphosate can also have a direct impact on non-target plants in theenvironments where it is used through spray draft or deliberate overspraying. This could lead to the loss of rare or endangered species oran overall reduction in diversity and numbers. Research carried out inthe UK on the use of glyphosate on GM RR beet showed significantindirect effects of this form of weed control. These included reducedweed numbers in arable fields and reduced weed seed production bothof which are potentially deleterious to species further up the food chain,including threatened bird species, if repeated over a number of years.It is apparent that glyphosate and its formulated commercialproducts (e.g. Roundup) can be harmful to species at manystages along the food chain, including the aquatic food chain.Regulators must ensure that usage of herbicides is safe forwildlife when it is used for purposes it has been approved for.Therefore, the safety of glyphosate to biodiversity urgentlyneeds to be re-assessed.
Glyphosate impacts on the soil-plant systemThe impact of glyphosate on soil biodiversity and the soil-plantsystem is of concern because of the effects observed with GM RRcrops. Glyphosate enters the soil by being directly sprayed on it, viathe roots of plants that have been sprayed, or from dead vegetation.Importantly, glyphosate affects the rhizosphere – the region of the soilsurrounding the roots that is essential to the health and nutrient uptakeof the plant. Surprisingly, the approvals processes for glyphosate andits formulated products around the world, including the EU, currentlydo not require exhaustive testing of its soil impacts.Studies of earthworms exposed to glyphosate showed reducedgrowth rate, reduced cocoon hatching and behaviour to avoid treatedareas. Earthworms are vital to soil health so any adverse effect on themis likely to affect soil health.Independent researchers are now publishing studies showing thatglyphosate has an impact on key functions of the rhizosphere.These include:■■■■■■
Reduction in the uptake of essential micronutrients by cropsReduction in nitrogen fixation, resulting in reduced yieldsIncreased vulnerability to plant diseases
‘…If GM herbicide-tolerant beet were to be grown andmanaged as in the FSEs [UK Farm Scale Evaluations2000- 2003] this would result in adverse effects onarable weed populations, as defined and assessed bycriteria specified in Directive 2001/18/EC, comparedwith conventionally managed beet. The effects on arableweeds would be likely to result in adverse effects onorganisms at higher trophic levels (e.g. farmland birds),compared with conventionally managed beet’(ACRE 2004)
Such changes can have a direct impact on the health andperformance of crops. Plant diseases - such as take-all incereals, damping off, root rot and sudden death syndromein soya - are encouraged by the changes in soil biology andchemistry that glyphosate induces. These impacts are ofconcern to farmers and environmentalists and need to beaddressed urgently.
6GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Glyphosate and the plague of resistant weedsWhen glyphosate first appeared in the mid 1990s, weed resistance toherbicides as a result of GM RR crops was rarely discussed, althoughthe phenomenon of weed resistance to herbicides was well known.Now, 15 years later, weed resistance to glyphosate is one of the mostwell documented effects and is a major environmental concern of thecultivation of GM RR crops.Since the introduction of RR crops, there has been a dramatic increasein the number of weed species exhibiting glyphosate resistance.Glyphosate resistance has now been confirmed in over 20 species,with over 100 resistant strains identified, primarily in the Americas.Many scientists attribute this increase to the over reliance onglyphosate to control weeds in fields of GM RR soya, maizeand cotton.
These strategies add to the amount of herbicides being used thereforeincreasing the overall toxic burden from GM RR crops and continuethe industrial agriculture treadmill of herbicide usage and resistance.The development of more weeds with resistance to multiple herbicidesseems probable. The widespread nature of weed resistance, andthe additional herbicides required to control these weeds means thatMonsanto’s promise of cheaper and easier weed control with GM RRcrops has not been deliveredThe toxicological profiles for mixtures of herbicides are not clear.However,it is clear that GM RR crops have brought about anescalation in the pesticides ‘arms race’ with an increasing toxicburden on the environment and people.
ConclusionRecent studies demonstrate that glyphosate-based herbicides,such as Roundup, can have harmful effects to human health and theenvironment. Exposure of humans to glyphosate has been linkedto various health effects including reproductive effects, cancer andneurological effects. Glyphosate interacts with soil chemistry andbiology, resulting is a variety of impacts including reduced plantnutrition and increased vulnerability to plant disease. Glyphosatemay also leach into surface and groundwaters, where they maydamage wildlife and possibly end up in drinking water. Glyphosate andRoundup are far from benign herbicides and a review of their safety forhuman and animal health and for the environment is urgently needed.GM RR crops have greatly increased glyphosate usage, especially inthe Americas where they are primarily grown. Given the new evidenceof glyphosate toxicity, this of great concern. The rise in glyphosateresistant weeds is associated with GM RR crops, and the escalationin the ‘arms-race’ against these resistant weeds fuels concerns thateven more glyphosate will be used in the future with GM RR crops,in stronger formulations and possibly with additional herbicides. Thisfacet of GM herbicide-tolerant crops should be enough to lead to aban on their cultivation.GM herbicide-tolerant crops, as epitomised by GM RR crops,are not part of sustainable agriculture practices. They are partof an industrial agriculture system that involves large-scalemonocultures that depend on costly, polluting inputs such asherbicides. There is no doubt that there is an urgent need tofind sustainable solutions to agriculture. As the recent UN/World Bank global assessment of agriculture (IAASTD) recentlystated, ‘business as usual is no longer an option’ (IAASTD2009b). Sustainable solutions will not come from GM crops,and definitely not from GM herbicide-tolerant crops.
‘No-tillage corn and soybean production has beenwidely accepted in the mid-Atlantic region, favouringestablishment of horseweed. Within 3 years of using onlyglyphosate for weed control in continuous glyphosate-resistant soybeans, glyphosate failed to controlhorseweed in some fields. Seedlings originating fromseed of one population collected in Delaware were grownin the greenhouse and exhibited 8- to 13-fold glyphosateresistance compared with a susceptible population’(Van Gessel 2001)
Controlling glyphosate-resistant weeds in GM RR crops is now a majorproblem for farmers. Monsanto acknowledges this, and has publishedguidance on how to deal with the growing weed resistance problemsin GM RR crops. Monsanto’s recommended strategies include:■■
the use of either stronger formulations of glyphosate or of mixturesof glyphosate and other herbicides, e.g. the notorious 2,4-D – oneactive ingredient of Agent Orange, the defoliant used by the USArmy during the Vietnam; andproducing GM seeds with several herbicide tolerant genes (genestacking), which would allow other herbicides, in addition toglyphosate, to be sprayed over crops.
■■
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 20117
� GREENPEACE / BERNHARD NIMTSCH
GM Roundup Readycrops include oilseedrape or canola (pictured),soya, maize and cotton.Such crops do notcontribute to sustainableagriculture practices.
GM Roundup ReadyHerbicide tolerance and GM cropscrops have lead toWhy the world should beincreasingly intensiveuse of glyphosate,UpReady to Roundasglyphosatethey allow farmers tospray the herbicideover growing crops.
� GREENPEACE / RODRIGO BALÉIA
Scientific evidenceshows that glyphosatecan have immediateand long-term, directand indirect toxiceffects on plants andanimals, as well asindirect effects linked tothe changes it causesin the ecosystem.8GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
1] IntroductionGlyphosate - the active ingredient in many herbicides soldthroughout the world - has always been promoted as ‘safe’.But is it?Mounting scientific evidence suggests that there can be adverseimpacts on human and animal health, and the environment. The safetyof glyphosate is in serious doubt.The most well-known formulated herbicide based on glyphosateis ‘Roundup’, sold by the US-based agricultural biotechnologycorporation Monsanto, the world’s leading producer of glyphosate.Monsanto is also the leading producer of genetically modified (GM,also called genetically engineered, GE) seed, producing glyphosate-tolerant GM crops that are marketed as ‘Roundup Ready’ (RR).The glyphosate-tolerance of these crops subsequently leads toa widespread use of glyphosate-based products; thus, the closecorrelation between crop and herbicide is a major cause for concern.This report examines the increasing evidence on the impacts ofglyphosate-based products - and glyphosate’s main breakdownproduct aminomethylphosphonic acid (AMPA) - on health, theenvironment, biodiversity and farmers. It also looks at the use ofglyphosate or Roundup on GM RR crops: how its use in connectionwith these crops is resulting in widespread weed resistance; what thatmeans for future herbicide usage; and the wider considerations aboutGM herbicide-tolerant (HT) crops.This report comes at a time when the use of Roundup has increaseddramatically around the world. At the same time there is a growingbody of evidence indicating its harmful impacts.
1.1 Glyphosate: how does it work?Glyphosate is a water-soluble, broad-spectrum, non-selectiveherbicide that is absorbed by the leaves and transported to all partsof the plant, including the roots. It is therefore capable of completelykilling even deep-rooted plants, in contrast to other products - such asparaquat - which affect only the leafy part of the plant above ground.This property, combined with marketing campaigns promoting it as a‘safe’ product, has made glyphosate a very popular herbicide.A Monsanto employee discovered the herbicidal nature of theglyphosate molecule in 1970. Monsanto introduced the firstcommercial Roundup product (Monsanto 2005a), which usesglyphosate as the active ingredient, in 1974. It is claimed that Roundupis now used in 130 countries on 100 different crops (Monsanto 2005a).The enzyme EPSPS (5-enolpyruvylshikimate-3-phosphate synthase) ispresent in all plants, fungi and bacteria. Glyphosate chelates (or binds)manganese, making it unavailable to the EPSPS. Because manganeseis essential for EPSPS to work (Johal & Huber 2009), inhibiting it in thisway subsequently affects an essential biochemical pathway in plants,the shikimate pathway, leading to a shortage of vital molecules forbuilding proteins and causing the plant’s death.Because EPSPS is not found in animals, it is assumed thatglyphosate is relatively harmless to mammals, insects, fish and birds.However, independent research shows that this is not the case. Inaddition glyphosate breaks down in the natural environment to formaminomethylphosphonic acid (AMPA), which is very similar in chemicalstructure to glyphosate. There is evidence that AMPA can also haveimpacts on animal and human health, and the environment.On its own, glyphosate is not very effective as a herbicide. Therefore,it is marketed in formulated products mixed with other chemicalsknown as adjuvants or surfactants. These chemicals enable theherbicide to stick to foliage and allow the glyphosate molecule topenetrate the cuticle on the leaves and enter cells and the plant’scirculatory systems. Glyphosate is then transported to all parts of theplant, including the tips of the roots.So, when examining the impact of this herbicide on health and theenvironment, it is important to take AMPA and the adjuvants orsurfactants into account. The impacts of these chemicals - singularlyand in combination - are explored in this report.
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 20119
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
GM RR varieties allow farmers to spray the herbicide over the growingcrop, killing virtually all weeds without affecting the crop itself.Roundup is Monsanto’s top-selling range of herbicides, and allMonsanto’s sales pitch to farmers promised - and still does - simplifiedproducts contain glyphosate as the active ingredient. There are manyweed control and reducing the number of spray passes required,different formulations of the product sold under the same brand namesubsequently reducing the costs of weed control (Monsanto 2009a).around the world - for instance Roundup PowerMax and RoundupHowever, the reality is turning out to be different with increasingWeathermax, or under other brand names such as QuikPro1.health (Chapters 2 and 3), biodiversity (Chapter 5) and environmentalMonsanto’s patent on glyphosate ran out in 2000. However, Monsanto (Chapters 4 and 6) concerns and the development of weed resistancehad already secured markets for their glyphosate by introducing(Chapter 7).GM seeds – soya, maize, cotton and canola - that are specificallyGM RR crops are primarily grown in the Americas. In 2009, moreengineered to be glyphosate tolerant. All Roundup Ready seeds arethan 90% of the soya crop planted in the US was GM RR (NationalGM, as there are no conventional methods to produce herbicideAgricultural Statistics Service 2009). GM RR maize and cotton weretolerance to Roundup. These GM seeds have been marketed fromalso widely grown. While GM RR soya also dominates the soya cropthe mid 1990s onwards2as Roundup Ready (RR). Because Monsantoin Argentina and Paraguay, adoption of the technology elsewhere indoes not guarantee crop performance with non-Roundup-brandthe world has been met with less enthusiasm. In Brazil, take-up of GMherbicides, farmers are encouraged to use only Monsanto’s RoundupRR soya has been much slower, with 40% of the soybean crop beingon the GM crops rather than other brands of glyphosate herbicidenon-GM in 2009/10 (The Crop Site 2010). In Europe, no GM RR crops(Monsanto 2011).have so far been approved for cultivation.
1.2 GM crops: a perfect match for Roundup
2.00
1.80
1.60
1.40
Glyphosate rate per crop year
1.20
1.00
0.80
0.60
0.40
0.20
0.00199619971998199920002001200220032004200520062007
CottonYear
Soybean
Corn
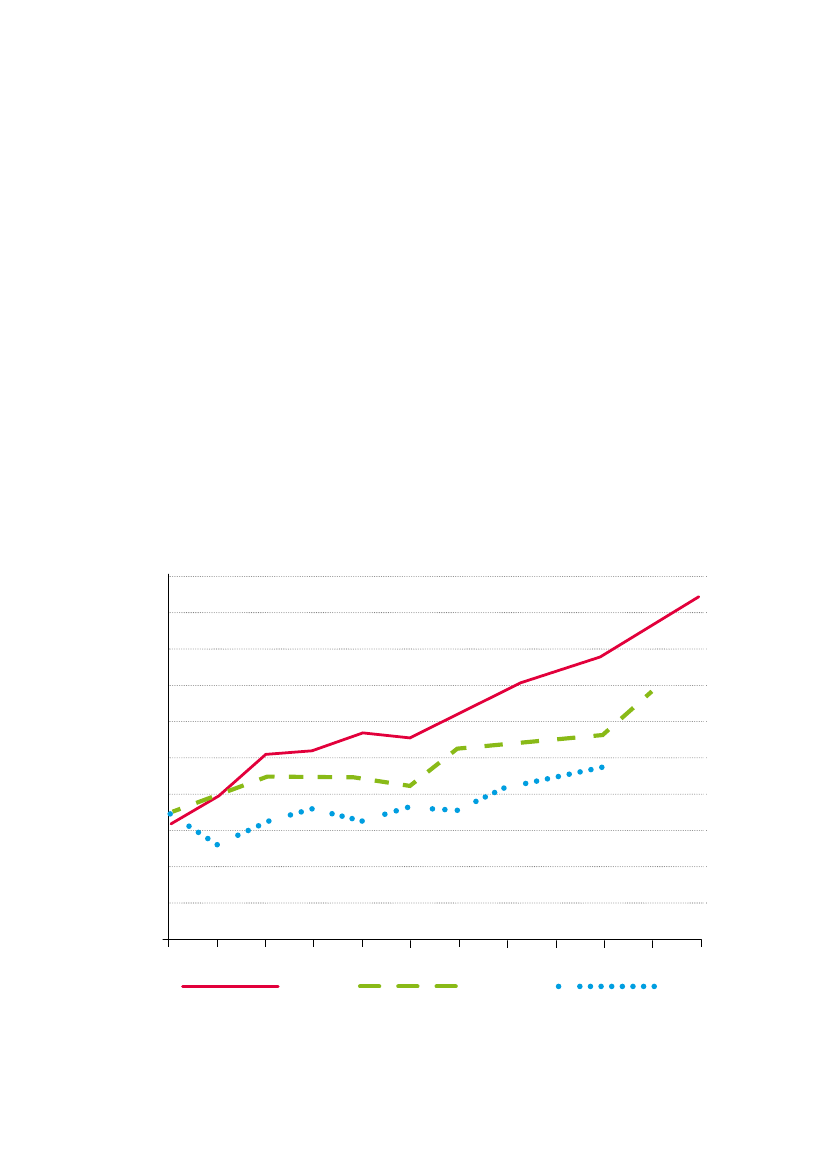
Fig. 1:Average glyphosate application rate per crop year for corn, soya and cotton in the US.Application rates of glyphosate-based herbicides haveincreased steadily since the introduction of glyphosate-tolerant RR cotton, soya and corn in the mid 1990s. Data are from US National Agricultural StatisticService who define “rate per crop year” as the average one-time rate of application multiplied by the average number of applications. Redrawn from Benbrook(2009) with permission.12http://www.monsanto.com/products/Pages/agricultural-herbicides.aspxhttp://www.cera-gmc.org/?action=gm_crop_database
10GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Since the introduction of GM RR seeds, the amount of glyphosateused in countries where these crops are grown has increaseddramatically. In a series of reports, Charles Benbrook analysedUSDA data charting the rise of glyphosate usage in the US since theintroduction of GM RR crops (Fig. 1; Benbrook 2001; 2004; 2009).He noted a 39% rise for maize (1996-2005); nearly 200% for cotton(1996-2007) and nearly 100% for soybean (1996-2006). Peer-reviewed literature also notes considerable increases of glyphosateassociated with the introduction of GM RR crops in the US (e.g.Duke, 2005; Cerdeira & Duke 2006). Similar trends have followedthe introductions of GM RR soya in Argentina (Binimelis et al. 2009)and Brazil (Lucas 2006). It is apparent that the introduction of GMRR seeds has been instrumental in the increased use of glyphosate -much of it as part of Monsanto’s Roundup range - in recent years.
1.3 Other uses of glyphosateThe potential markets for glyphosate extend beyond GM RR crops tomany types of arable crops and many types of land management (see,e.g. Monsanto 2005a).Increasingly, Monsanto is marketing GM insect-resistant crop varietiesthat also include glyphosate-tolerance (RR) genes3. In addition,crops with the GM RR gene and tolerance to other herbicides suchas dicamba are being developed by biotech companies to deal withglyphosate-resistant weeds (see Chapter 7) (Behrens et al. 2007;Service 2007; Stride 2010).
1.4 SummaryGlyphosate is the active ingredient in many herbicides. It is generallysold as formulations that include other ingredients in order to increaseits effectiveness by allowing it to adhere to plant leaves. The mostwell-known of these formulations is the Roundup range of herbicidessold by Monsanto. Although first marketed in 1974, glyphosate useincreased drastically following the introduction of GM RR crops in themid 1990s. Monsanto maintains a high market share of glyphosatesales by selling Roundup as a package with its GM glyphosate-resistant seeds.The dramatic increase in the use of glyphosate has seriousimplications for health and the environment. These implicationsare described in the following chapters.
Since the introduction of GM Roundup ready (RR) seeds,the amount of glyphosate used in countries where thesecrops are grown has increased dramatically.
3
http://www.monsanto.com/products/Pages/cotton-seeds.aspxGM Freeze and Greenpeace| GRL-TN 03/2011 |June 201111
� GREENPEACE / DANIEL BELTRÁ
Glyphosate is presentin soils, waters and ourfood as a result of itswidespread use with GMRoundup Ready crops.
Aerial spraying of RRHerbicide tolerance and GM cropscrops such as soyaWhy theexposure ofbeincreasesworld shouldpeopleto Round UpReadyto glyphosate-glyphosatebased herbicides.
� GREENPEACE / DANIEL BELTRÁ
Since the early days of theircommercialisation, glyphosateand Roundup have beenmarketed as ‘safe’ or benign.Yet increasingly, the scientificliterature indicates that theseproducts are far from beingsafe. Independent scientificstudies are now providingdetails of Roundup’s effects,especially its chronic effects onhuman health.12GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
2] Glyphosate impacts on human healthGlossary of termsAxonthe long fibre of a neuron.Congenitalpresent at birth.Dendritesthreadlike extensions of the cytoplasm of a neuron.Dopaminea chemical produced by the brain, which functions asa neurotransmitter.Free radicalselectrically-charged reactive atoms or moleculesin cells, which can damage other molecules within cells.Globus palliduspart of the nucleus of the brain.Implantationthe attachment of the embryo to the lining ofthe uterus.Lymphocytesvertebrate white blood cells.Necrosispremature death of cells.Mitochondriacell organelles responsible for energy production.Mitochondrial transmembrane potential- difference in voltageacross a mitochondrial membrane.Mitochondrial energyenergy generated by mitochondria in cells.Ossificationthe process of creating bone.Oxidative stressvarious pathologic changes seen in livingorganisms in response to excessive levels of free radicals inthe environment.Plasmathe fluid portion of the blood.RNA transcriptionthe synthesis of RNA from a DNA template.Serotonina chemical produced by the brain, which functionsas a neurotransmitter.Steroidogenic acute regulatory (StAR) proteinprotein thatregulates steroid hormone synthesis.Seminal tubulestubes that carry sperm from the testes.Substantia nigramovement centre in the brain.
2.1 Chronic effects2.1.1 ReproductiveBirth defects in the Argentinean state of Chaco, where GM soy andrice crops are heavily sprayed with glyphosate, increased nearlyfourfold over the years 2000 to 2009, according to a report released bythe Chaco state government in April 2010 (Otaño et al. 2010). Paganelliet al. 2010 also reported ‘several cases of malformations togetherwith repeated spontaneous abortions were detected in the village ofItuzaingo’ Cordoba, which is surrounded by GMO-based agriculture’.On its own, this information does not implicate glyphosate, for otherpesticides are also used on the soy and rice fields. However, takentogether with laboratory studies and other epidemiological information(patterns of illness in the human population), it raises concerns that canno longer be ignored.
‘…Several cases of malformations together with repeatedspontaneous abortions were detected in the village ofItuzaingo’ Cordoba, which is surrounded by GMO-basedagriculture’.(Paganelli et al. 2010)
In Paraguay, 52 women who were exposed to glyphosate-basedherbicides during pregnancy delivered offspring with congenital (i.e.present at birth) malformations. These birth defects showed strikingsimilarities to those induced by glyphosate in laboratory experiments(Paganelli et al. 2010). However, they cannot yet be linked directly toglyphosate exposure.The congenital malformations included microcephaly (small head),anencephaly, and cranial malformations. Anencephaly occurs whenthe neural tube fails to close during pregnancy, resulting in the absenceof the majority of the brain, skull and scalp.
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201113
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
In 2009, Argentinean researchers lead by Professor Carrasco showedthat even weak concentrations (down to 0.02%) of a commercialglyphosate formulation caused disruption to the development of thecraniofacial skeleton of tadpole embryos (Fig 2). Other effects includedshortening of the trunk, reduced head size and eye defects. The authorsconcluded that their results were ‘compatible with the malformationsobserved in the offspring of women chronically exposed to glyphosate-based herbicides during pregnancy’ (Paganelli et al. 2010). Althoughthis study has recently been criticised by the agrochemical industry(Saltmiras et al. 2011), the study nonetheless raises concern regardingthe impacts of glyphosate on reproduction.CONTROLGBH-TREATED
Previous studies also indicate the potential of glyphosate todisrupt reproduction. One study showed that applying glyphosateand Roundup at dilutions far below those used in agricultureseverely affected human embryonic and placental cells, producingmitochondrial damage and two types of cell death, necrosis andprogrammed cell death, within 24 hours. Cell deaths occurred atconcentrations corresponding to the level of residues in food expectedfrom Roundup-treated GM crops (Benachour & Séralini 2009). Theauthors concluded that, if this occurred in the body, it would resultin impacts on fertility, as well as carbohydrate metabolism, immunesystem function and water balance.
‘Applying glyphosate and Roundup at dilutions farbelow those used in agriculture severely affected humanembryonic and placental cells, producing mitochondrialdamage and two types of cell, death necrosis andprogrammed cell death, within 24 hours.’(Benachour & Séralini 2009)CONTROL
Other studies demonstrate glyphosate and/or Roundup’s endocrinedisrupting effects:■■
Roundup disrupted the production of the femalereproductive hormone progesterone in mouse cells bydisrupting expression of the steroidogenic acute regulatory(StAR) protein(Walsh et al. 2000).Glyphosate at dilutions 100 times lower than agriculturalrates inhibited activity of the enzyme aromatase, which isresponsible for synthesis of another female reproductivehormone oestrogen.Roundup itself had an even greater effect.This effect occurred once the glyphosate and Roundup had enteredthe cells, but prior to entry Roundup had the opposite effectcausing 40% increase in aromatase activity (Richard et al. 2005).The authors concluded this might explain premature births andmiscarriages observed in female farmers using glyphosate (Savitz etal. 1997; Arbuckle et al. 2001).Hokanson et al also demonstrated a synergistic effect ofglyphosate with oestrogen, with implications for pregnancy-induced hypertension and foetal growth retardation.(Hokanson et al. 2007)In 2007, Benachour et al. demonstrated that low levels ofglyphosate inhibit aromatase in human embryonic cellsresulting in reduced oestrogen production, with adjuvantsin Roundup increasing the effect.(Benachour et al. 2007)
GBH-TREATED
■■
CONTROLIMAGEPAGANELLI ET AL. 2010
GLYPHOSATE-INJECTED
■■
■■
Fig 2:Glyphosate-based herbicides (GBH), and glyphosate itself,interfere with early development in both frog and chicken embryos(Paganelli et al. 2010).Clear differences in frog embryos are seen here, withmalformations present in those exposed to a 1/5,000 (0.02%) dilution of GBHbut note, the last panel are injected with glyphosate.Reproduced with permission from American Chemical Society. For full details,see Paganelli et al. (2010)
14GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
■■
Roundup, but not glyphosate, inhibited the conversionof androgens to oestrogen.However glyphosate was anti-androgenic at levels 40 times lower than residues permitted insoybeans (Gasnier et al. 2010).Pre-pubertal exposure to the product Roundup Transorbdelayed puberty, altered the structure of seminal tubules inthe testes of male rats, and reduced testosterone production(Romano et al. 2010).
Other ways in which glyphosate may be contributing to cancer include:■■
■■
its ability to deregulate cell division,a hallmark of tumour cells,demonstrated to occur in sea urchin embryos at concentrations upto 4,000 times lower than normal sprayed concentrations (Marc etal. 2002, 2003, 2004);its inhibition of RNA transcription,demonstrated in sea urchinembryos at concentrations 25 times lower than normal sprayedconcentrations (Marc et al. 2005); andits ability to cause oxidative stress,demonstrated forglyphosate and/or Roundup in human lymphocytes (Lioi et al1998a; Pieniazek et al 2004) and skin cells (Gehin et al. 2005;2006), as well as in bovine lymphocytes (Lioi et al. 1998b), bullfrogtadpoles (Costa et al. 2008), pregnant rats and their foetuses(Beuret et al. 2005), rat liver cells (El-Shenawy 2009), mouse kidneycells and liver DNA (Bolognesi et al. 1997), and in rice leaves (Ahsanet al. 2008).
■■
The implications of these effects on reproduction and the developingfoetus are profound, with work by Mose et al. 2008 confirming thatglyphosate does cross the placenta.Finally, high-dose experiments on rats resulted in decreased totalimplantations and viable foetuses, reduced litter size, reduced foetalweight and pup weight, and reduced ossification of the breastbone(US EPA 1993, 2006; IPCS 1994).2.1.2 CancerThe Chaco report (Otaño et al. 2010) mentions a significant increasein cancer and particularly child cancer including leukaemia, lymphomaand brain tumours. Once again, while these could be caused by anumber of factors including other pesticides, there is support fromepidemiology and laboratory studies to indicate that glyphosate mightbe contributing to these cancers.A number of epidemiological studies have linked exposure toglyphosate tonon-Hodgkin’s lymphoma(Norsdtrom et al. 1998;Hardell & Eriksson 1999; Hardell et al. 2002; McDuffie et al. 2001;De Roos et al. 2003; Eriksson et al. 2008;) andmultiple myeloma(De Roos et al. 2005). Three studies of people exposed to the aerialspraying of illegal crops in Columbia have foundDNA damageamongst those who had experienced acute effects from the spray(Mueckay & Malondao 2003; Paz-y-Mino et al. 2007; Bolognesi et al.1997).A number of laboratory studies have shown glyphosate, Roundupand/or the metabolite of glyphosate, AMPA, to begenotoxic ormutagenicin human cells, including liver (Mañas et al. 2009a;Gasnier et al. 2010; Mañas et al. 2009b), and lymphocytes (Lioi etal. 1998a; Bolognesi et al. 1997; Vigfusson & Vyse 1980). Numerousother studies have demonstrated genotoxicity or mutagenicity inmouse, bovine, fish, caiman, tadpole, fruit fly, sea urchin, onion andbacterial cells (Rank et al. 1993; Kale et al. 1995; Bolognesi et al. 1997;Clements et al. 1997; Peluso et al. 1998; Lioi et al. 1998b; Kaya etal. 2000; Grisolia 2002; Siviková & Dianovský 2006; Bellé et al. 2007;Cavaş & Könen 2007; Cavalcante et al. 2008; Guilherme et al. 2009;Mañas et al. 2009b; Mañas et al. 2009a; Poletta et al. 2009).
■■
2.1.3 NeurologicalGlyphosate may affect the nervous system and may even beimplicated in neurodegenerative diseases such asParkinson’sdisease.Both Roundup and glyphosate were found to inhibit growthof ‘neurite-like structures’ (axons or dendrites), at concentrationslower than those measured in plasma and tissue of farmersexposed to Roundup (Axelrad et al. 2003). Two other studies onrats have demonstrated that glyphosate depletesserotonin anddopamine(Anadón et al. 2008); and caused a loss of mitochondrialtransmembrane potential in rat brain cells, especially in thesubstantianigraregion of the brain (Astiz et al. 2009). The brain is very dependenton mitochondrial energy to maintain normal physiology, andlossof mitochondrial functionis associated with many humanneurodegenerative disorders. Damage in thesubstantia nigraisimplicated in Parkinson’s disease. Additionally, the central nervoussystem - and particularly thesubstantia nigra- is highly sensitive tofree radical damage, which results fromoxidative stress.A numberof studies reported earlier show that glyphosate and Roundup causeoxidative stress in various different cells, including brain cells.These laboratory findings are reflected in an epidemiological studyand one reported clinical case. In a study of children born to pesticideapplicators in Minnesota in the US, 43% of the children reported tohave ADD/ADHD (Attention Deficit Hyperactivity Disorder) had parentswho were exposed to glyphosate-containing herbicides (Garry et al.2002). A 54-year-old man developed skin lesions six hours after heaccidentally sprayed himself with a glyphosate herbicide, and onemonth later developed a ‘symmetrical Parkinsonian syndrome’. Twoyears later, magnetic resonance imaging revealed effects in theglobuspallidusandsubstantia nigraregions of the brain associated withParkinson’s disease (Barbosa et al. 2001).
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201115
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
2.2 Acute effectsA number of deaths have resulted from intentional ingestion (suicide),preceded by metabolic acidosis, respiratory and kidney failure, cardiacarrest, seizures, and coma (IPCS 1994; Chang & Chang 2009).The most commonly reported acute effects from occupational andbystander exposures to glyphosate-based herbicides are those ofthe skin, eyes, respiratory, gastrointestinal and cardiac systems. Theyinclude:■■
2.3 SummaryChronic effects related to glyphosate and its derivative products canbe classified in the following categories:reproductive(birth defects),cancer, neurological(even implicated in causing Parkinson’sdisease), andacute effectslinked with the direct use of the productby farmers or exposure of bystanders.There is concern that birth defects experienced by women in Argentinaand Paraguay may result from their exposure to glyphosate usedon GM soya and rice crops. Other studies have demonstratedglyphosate’s potential to disrupt reproduction by its ability to causemitochondria damage, necrosis and cell death in human embryonicand placental cells; and to cause endocrine disruption, includingdisruption of progesterone and oestrogen production, and delayedmale puberty.
irritation, swelling, tingling or burning of the skin, dermatitis, photo-contact dermatitis;conjunctivitis, painful eyes, corneal injury, burning eyes, blurredvision, double vision, swelling of the eye and lid;oral and nasal discomfort, unpleasant taste, tingling and irritation ofthroat, sore throat, swollen tongue;
■■
■■
Epidemiological studies have linked exposure to glyphosate with non-Hodgkin’s lymphoma and multiple myeloma, as well as DNA damage■■burning in chest, cough;among people who had experienced acute symptoms from glyphosateexposure. These findings are supported by laboratory studies that■■nausea, vomiting, headache, fever, diarrhoea, shakes and chills,demonstrate that glyphosate can cause genotoxicity, mutagenicity,tiredness, lethargy; andoxidative stress and dysregulation of cell division. Potential chronic■■rapid heartbeat, raised blood pressure, dizziness, light-headedness,neurological effects include Parkinson’s disease and ADD/ADHD,tingling in hands and feet; aching arms (IPCS 1994; Goldstein et al.while acute exposure symptoms include a wide range of effects on2002; Bradberry et al. 2004).skin, eyes, respiratory, gastrointestinal and cardiac systems.One recent epidemiology study in the US reported that exposure toThese effects must be taken very seriously and an urgentglyphosate herbicides was associated with both asthma and rhinitisreassessment of the health impacts of glyphosate and its(‘runny nose’) (Slager et al. 2009).related products must now take place.Significant exposure to glyphosate herbicides has occurred in Ecuadorand Columbia as a result of the aerial spraying campaign to eradicatecoca in Columbia and along its border with Ecuador since 1997.Symptoms reported there include many of those reported aboveand, in addition, red eyes, skin rashes and blisters, skin infections,abdominal pain, gastrointestinal infections, respiratory infections,difficulty in breathing, numbness, insomnia, depression, debilitation,weeping eyes (Gallardo 2001; Oldham & Massey 2002; Paz-y-Miño etal. 2007).
16GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Aerial spraying of glyphosate.
Glyphosate is toxic to somefrogs and their tadpoles,which may affect otherparts of the food chain.
Glyphosate residues arefound in our food.
Gly
Gly
Gly
FO
OD
Gly
More and moreweeds are becomingresistant to glyphosateresulting in increasedapplications andconcentrations ofglyphosate, andadditional herbicides.
GM herbicidetolerant cropsencouragemonoculture.Fewer weedsmeans lessfarmlandbiodiversity.
Glyphosate enters streamsand watercourses. Glyphosateand its breakdown product,AMPA can leach through thesoil and pollute groundwater.Contamination of waters withglyphosate can affect aquaticsystems and drinking water.
Glyphosatekills non-targetnative plantsalong field edgescausing losses inbiodiversity.
Exposure toglyphosate is linkedwith various healtheffects, includingcancer and damage tothe nervous system,and is a suspectedendocrine disruptor.
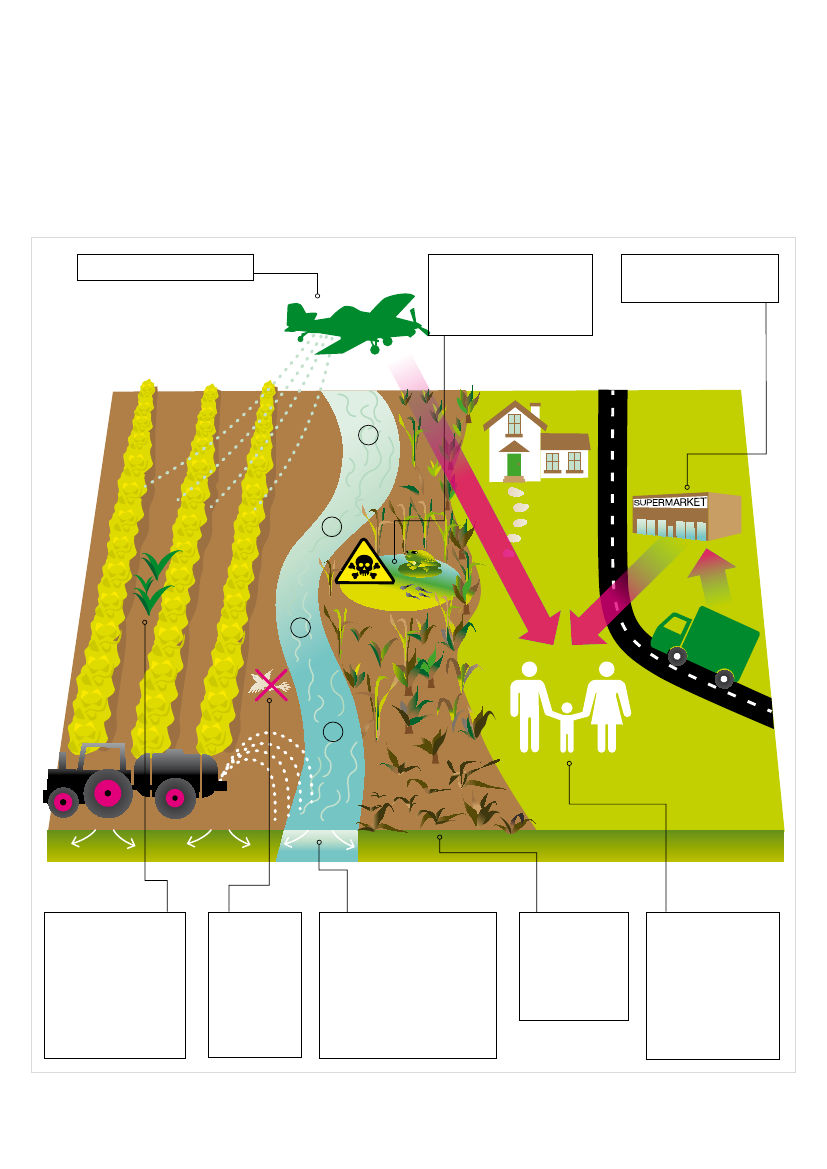
Fig 3:Environmental and human health effects of glyphosateGM Freeze and Greenpeace| GRL-TN 03/2011 |June 201117
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
An urgentassessment of thehealth impacts ofglyphosate and itsrelated productsmust now takeplace.
� GREENPEACE / GUSTAVO GILABERT
18GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
3] Glyphosate residues in foodThe use of Roundup on food and feed crops means that residuesof glyphosate and other chemicals used in the various formulationswill be found in our food (Fig. 3). However, data on the presence ofglyphosate and its breakdown product aminoglyphosate acid (AMPA)in food, feed and animal products from glyphosate sprayed crops aresparse.Maximum Residue Levels (MRLs) are the maximum permittedconcentration of pesticide residue in a food or animal feed. MRLs areprimarily trading standards, but they are also intended to ensure thatpesticide residues do not pose a risk for consumers. The current MRLsfor residues of glyphosate in food were agreed by the United NationsFood and Agriculture Organisation’s Codex Alimentarius (or food code)in 2006 and are listed in Table 1. The MRLs are for the combined levelsof both the herbicide and its main breakdown product, AMPA.Glyphosate is frequently used to desiccate cereal and oilseed rapecrops immediately prior to harvesting. This results in residues in cropsand processed products. The MRLs in Table 1 are generally higherfor crops where glyphosate is applied directly than when it is used forweed control prior to sowing. That is, MRLs are higher for crops whereglyphosate is used as a desiccant to dry grain prior to harvest (e.g.wheat and barley) or for crops where GM RR crops are commerciallygrown (e.g. soya, maize, cotton, rapeseed), than those for crops (e.g.pea and bean) where glyphosate is not sprayed on the crop itself, butmay be used for clearing a field before planting. Thus, MRLs appearto be based upon the levels likely to be found in a specific productas a result of expected usage of glyphosate, rather than on safetyconcerns.Table 1:Maximum Residue Levels (MRLs) for glyphosate in foods4CommodityAnimal productsPoultry meatMeat (from mammals other than marine mammals)Edible offal of poultryEdible offal of pigsEdible offal mammalian (except pigs)EggsCropsBananaBeans (dry)Sugar canePeas (dry)MaizeSunflower seedSugar cane molassesSoya bean (dry)Wheat bran unprocessedRape seedCereal grainsCotton seedSorghum straw and fodder. DryOat straw and fodder. DryMaize fodder. DryBean fodder. DryWheat straw and fodder DryBarley straw and fodder. DryHay or fodder (dry) of grassesAlfalfa fodderPea hay or pea fodder (dry)0.052.02.05.05.07.0102020203040501001502003004005005005000.050.050.50.55.00.05MRL mg/kg
4Source: UN FAO Codex Alimentarius. http://www.codexalimentarius.net/pestres/data/pesticides/details.html;jsessionid=7BA965F7D5BAA909CE2C7C2A6E3FAF97?d-16497-o=2&id=158&d-16497-s=3
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201119
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Sampling of foodstuffs often detects glyphosate and/or AMPA.■■
In Denmark, sampling of cereals in successive years in the late1990s found glyphosate and/or its degradation product AMPA inmore than half of the cereal samples. The average concentrationof glyphosate in 46 samples from the 1999 harvest was 0.11 mg/kg compared with 0.08 mg/kg in 49 samples for the 1998 harvest(Granby & Vahl 2001).In the UK, sampling of food for glyphosate residues has largelyconcentrated on cereals, including bread and flour. Glyphosatehas been regularly detected, and usually below the current MRL(Pesticides Residues Committee 2010, 2009, 2008 and 2007a). In2006, the UK’s Pesticide Residues Committee monitoring found asample of wheat flour containing 0.8 mg/kg above the Codex MRLof 0.5 mg/kg (Pesticide Residues Committee 2007b).Residues of glyphosate in tofu and soya pieces were reported in theUK in 2006. Six out of eight samples of tofu/soya pieces originatingfrom Brazil contained glyphosate with the highest level recodedbeing 1.1 mg/kg (Pesticide Residues Committee 2007a).In an EU survey of pesticide residue frequency, glyphosate wasfound in 9.54% of samples in 2007 (EFSA 2009).
■■
■■
Fig 4:Food label displaying RR soya ingredients
■■
SummaryDespite the extensive use of products that contain glyphosate,there are limited data on residues in food and feed, including animalproducts such as offal, consumed by people and animals. However,there are data showing that glyphosate and AMPA are found in fooddestined for people at levels below the current MRLs.The MRLs do not appear to be based on whether a specific residuelevel is safe or not but more on the levels likely to be found in a specificproduct as a result of agricultural practice, e.g. the use of glyphosateas a dessicant. As recent scientific studies (see Chapter 2) questionthe human safety of glyphosate-based products, the basis upon whichthese MRLs are made is called into question.Along with a rigorous review of the environmental and thehealth impacts of glyphosate, a revision of the existingMRLs is also needed. In light of the new scientific evidenceon glyphosate impacts it is essential to re-evaluate MRLs inorder to ensure that they remain in line with updated safetyassessments.
WHO/FAO 2005 reported on feeding trials in pigs that were given feedcontaining 40, 120 and 400 mg/kg of glyphosate and AMPA. At thehighest level (400 mg/kg), glyphosate residues in the liver were 0.72(1.4 including AMPA) mg/kg and in kidneys, 9.1 (11) mg/kg. Theseresidues are comparable with the MRL for edible offal from pigs of 0.5mg/kg (Table 1). Despite this, no animal products have been sampledin the EU in recent years (EFSA 2009) nor in the US (USFDA 2008),meaning that exposure of consumers has not been monitored inrecent times.The levels found in cereals and animal products are below the currentMRLs but indicate that consumers of cereal-based products areregularly exposed to glyphosate and AMPA residues. Importantly,the MRLs seem more dependent on the levels likely to be found in aspecific product rather than on whether a specific residue level is safeor not.
20GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
CREDIT: PETE RILEY
In light of newscientific evidenceon glyphosateimpacts, it isessential to re-evaluate maximumresidue levels inorder to ensure thatthey remain in linewith updated safetyassessments.GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201121
� GREENPEACE / GUSTAVO GILABERT
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
� GREENPEACE / GUSTAVO GILABERT
“…Glyphosate,when applied in lateautumn, can leachthrough the rootzone at unacceptableconcentrations inloamy soils”(Kjær et al. 2003)
22GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
4] Glyphosate in water“From soil and plant applications of glyphosate herbicide,it is expected that a small amount of the appliedglyphosate may enter surface waters through runoff orattached to soil particles that wash off treated field”(Monsanto 2003)Glyphosate has often been detected during monitoring of surfacewaters and groundwater. A comprehensive study of streams inthe midwest US examined the presence of herbicides, includingglyphosate and AMPA, at different stages in the crop-growing cycle(Battaglin et al., 2005). Glyphosate was detected during every seasonup to a maximum concentration of 8.7 μg/l. This is over 80 times theEU maximum permitted concentration of 0.1 μg/l in drinking water(European Union Council 1998) but substantially below the US drinkingwater maximum concentration of 700 μg/l (US EPA 2009). Sucha massive difference in permitted concentrations is hard to justify,especially given the growing body of evidence on the harm glyphosatecan cause to health and aquatic life. AMPA was also detected above0.1 μg/l in more than half of the samples taken through the year,most frequently after crops had emerged from the soil. The maximumconcentration of AMPA recorded in this study was 3.67 μg/l.
Glyphosate is highly soluble in water and therefore has the capacity tobe mobile in aquatic systems. In fact, glyphosate is far more soluble (inthe range 10 000-15 700 mg/l at 25oC) than other herbicides, such asatrazine (in the range 20-35 mg/l) and isoproturon (in the range 70-72mg/l), which are already known to leach from the soil to contaminatesurface waters. However, as will be discussed further in Chapter 6, itis glyphosate’s capacity to bind tightly to soil particles that prevents itfrom being highly mobile. Binding can immobilise it in the soil providedthat there are sufficient suitable sites. This varies depending on the soiltype and composition. Studies have found that binding of glyphosateis greater in soils with lower pH (i.e. more acidic) (Gimsing et al. 2004)and that phosphates (Simonsen et al. 2008) can compete for bindingsites. All of this adds to the complexity of glyphosate’s movements inthe soil, and predictions of its leachability.
Further evidence that glyphosate can enter surface waters comesfrom monitoring in Alberta, Canada, where it was found in 8 out of13 sites and in 22% of samples taken in wetland and streams, with apeak concentration of 6.07 μg/l (Humphries et al. 2005). In Denmark,a major government-sponsored study on the leaching of pesticideswas undertaken between 1999 and 2009. The conclusions of aninterim report were that ‘glyphosate, when applied in late autumn, canA report by the World Health Organisation (WHO 2005) confirmed thatleach through the root zone at unacceptable concentrations in loamyglyphosate is found in surface waters at levels between 0.5 μg/l and 1soils’ (Kjær et al. 2003). Glyphosate was detected to the depth of theμg/l and its environmental breakdown product, AMPA, was present atdrainage system and not in groundwater (Kjær et al. 2003, 2005).levels around 6 μg/l5. The levels of glyphosate exceed the maximumThe final report (Rosenbom et al. 2010) declared that glyphosate andallowed for pesticides in drinking water under EU law (see below) andAMPA exhibited ‘pronounced leaching’. A peer-reviewed paper basedwould require water companies to undertake expensive filtration before on the study stated ‘both glyphosate and AMPA can leach throughthe water could be supplied to the public.structured soils, they thereby pose a potential risk to the aquaticenvironment’.
5
In the Netherlands in 1988/89
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201123
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
The Danish study monitored pesticides leaching from different soiltypes, crops/agronomy and climates. Loamy soils (with roughlyequal amounts of sand, silt and clay) were found to be more proneto leaching of glyphosate and AMPA than coarse sandy soils, wherematrixes of aluminium and iron provide the right conditions for sorptionand degradation. On loamy soils, autumn application resulted indetectable concentrations of glyphosate and AMPA in the drainagewater in the upper metre of soil, often at concentrations exceedingthe EU’s maximum concentration for drinking water. The maximumconcentrations of glyphosate recorded in drainage water at thetwo most vulnerable sites were found in 2009 (31 μg/l and 4.7 μg/lrespectively). Average concentrations of glyphosate in drainage waterfollowing the first drainage after application were well above 0.1 μg/lfor some crops, for instance maize in 2005 (4.04 μg/l) and peas in2001 (0.54 μg/l), both following the application RoundUp. Detection ofglyphosate and AMPA was mainly confined to drainage water althoughit was detected at three sites below the drainage system. At one sitein the wet August of 2008, glyphosate was frequently detected ingroundwater, with a maximum concentration of 0.67 μg/l.The Danish results show that, in certain soil types with low sorptioncapacity, glyphosate can easily find its way into surface waters atconcentrations that well exceed the EU drinking water maximum of0.1 μg/l. In more exceptional circumstances, i.e. after heavy rain, it canalso find its way into groundwater.Standards for protecting aquatic life from glyphosate have not beenwidely set. None are agreed in the US or the EU, for example. InCanada, an interim standard of 0.65 μg/l was agreed upon as longago as 1989 (Canadian Council for Environment Ministers, 1999) andwork is presently in progress to establish a new one. US researchersinvestigating small water bodies in areas where glyphosate-basedherbicides were used found levels up to 328 μg/l, well above the levelset in Canada to protect freshwater aquatic life (Battaglin et al. 2008).The amount of glyphosate entering watercourses is dependent onthe weather immediately following its application. Heavy rain onlow sorption soils is most likely to result in glyphosate washing intodrainage systems.
Glyphosate can enter surface waters from land-based spraying eitherby becoming attached to soil particles, by leaching or by spray driftat concentrations that, in the EU, would have to be removed beforewaters entered the public supply. For example, small catchmentstudies in Sweden (Keuger 2005), France (Delmas 2004) and Greece(Papadopoulou-Mourkidou 2004) have confirmed that glyphosate canleach into drainage systems and surface waters. Losses amount to asmall percentage of the glyphosate applied in the catchment but canexceed levels permitted in drinking water.The use of glyphosate on paved surfaces in urban settings can alsoresult in glyphosate quickly entering drainage water - and hence,surface waters - immediately after rainfall. A study in France showedthat glyphosate can enter watercourses more readily from urban areasvia the sewerage system than in rural environments due to applicationson roads and railways. High levels were linked to rainfall events (Bottaet al. 2009). Glyphosate is banned from use on hard surfaces inDenmark and by half of Swedish municipalities (Kristoffersen 2008).
ConclusionGlyphosate is mobile in the root zone in soils with weak sorptioncapacity. This results in the presence of glyphosate and its degradationproduct, AMPA, in drainage water and surface waters. Groundwaterhas been polluted in similar soils after spraying followed by heavyrainfall. Run-off from the weed treatment of paved areas can alsocontribute to levels of glyphosate in watercourses.These finding have implications for surface water quality anddrinking water quality. The leaching of glyphosate also hasimplications to aquatic life given the evidence that glyphosatecan cause harm to health and the environment(see Chapters 2and 5).
24GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
The leaching ofglyphosate alsohas implicationsto aquatic lifegiven the evidencethat glyphosatecan cause harmto health andenvironment
� GREENPEACE / JIRI REZACGM Freeze and Greenpeace| GRL-TN 03/2011 |June 201125
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
5] Glyphosate impacts on biodiversityAlthough promoted as a benign herbicide, accumulated scientificevidence detailed in this chapter shows that glyphosate, and itsformulated commercial products such as Roundup, can affectbiodiversity.Glyphosate can impact on plants and animals via:■■■■■■
In the past, testing pesticides on amphibians, as part of the approvalprocess, was rare. When it did occur, it was only over short time periods(Reylea 2005a). However, the global decline of amphibian numbersled researchers to focus on agro-chemicals as a potential cause oftheir decline. Glyphosate and Roundup formulations were selected forindependent toxicity studies because of their widespread use.The conclusions from several projects suggest that, under close-to-field-conditions, glyphosate-based products, including Roundup, havea direct toxic effect on the adults and tadpoles of a range of amphibianspecies:■■
direct toxic effects of exposure to the spray;chronic effects caused by long-term exposure in the ecosystem; andindirect effects due to changes in the ecosystem.
5.1 Direct toxic effectsSince Roundup was first introduced in the 1970s, Monsanto hasconsistently claimed that glyphosate and Roundup are not likely toharm animals. It argues that, because glyphosate destroys an enzymein plants that is not present in animals, it will not affect them.For example, Monsanto says ‘glyphosate-containing products labelledfor forestry use have shown no adverse effect on aquatic animals’(Monsanto 2010a) and that these products present ‘extremely lowtoxicity to mammals, birds and fish‘ (Monsanto 2010b).However, there is now a significant body of evidence from the peer-reviewed scientific literature showing that these claims can no longerbe supported where Roundup formulations are applied. The toxicityof glyphosate is strongly increased by the adjuvants and surfactantsthat it is mixed with in order for it to adhere to foliage and penetrateinto plant cells, allowing it to then be transported (or translocated) toall parts of the plant. Approvals of products are based on separateassessments of glyphosate and the adjuvants and surfactants6butnot the combined commercial product. At least 12 different adjuvantshave been used in glyphosate-based formulations (Cox 2004). In mostcases, the mixtures and ratios are commercially confidential.5.1.1 Toxicity to amphibiansDeclines in the numbers and the diversity of amphibian species acrossthe world have been widely reported since the 1980s. It is estimatedthat one in three of species globally is threatened with extinction(Williams 2004). Causes such as habitat loss, habitat fragmentation,disease and environmental contamination have been put forward ascontributing to this decline.
Roundup was found to have the potential to cause substantialmortality in many amphibian species in a controlled study of aquaticcommunities that included algae and tadpoles from five NorthAmerican species of toads and frogs (Reylea 2005b).Three species of North American frog and toad tadpoles exposedto Roundup in artificial ponds exhibited very high mortality (96-100%) over three weeks, which the author suggests could lead topopulation decline in the wild (Reylea 2005c).Western chorus tadpoles exposed to the glyphosate productRoundup WeatherMax at 572 �g/l glyphosate acid equivalents (a.e.)resulted in 80% mortality, which the authors suggested resultedfrom a unique surfactant formulation. Exposure to WeatherMax orRoundup Original Max at 572 �g/l a.e. also lengthened the larvalperiod for American toads (Williams & Semlitsch 2010).Frog tadpoles (Rhinellaarenarum)exposed to concentrations usedin commercial formulations showed decreases in the activities ofAChE (acetylcholinerterase) (Lajmaonovich et al. 2010).
■■
■■
■■
The findings of these studies suggest that glyphosate-based productsharm amphibians at concentrations which occur as a result of theirnormal use in agriculture or forestry. This group of animals includesmany species that are predators of pests in and around agro-ecosystems and forest ecosystems. Losses of the order reportedabove in wild populations could have significant impacts upon pestpopulations and a long-term impact on crop yield and quality.
6
Adjuvants have been categorised as extenders, wetting agents, sticking agents and foggingagents designed to enhance the activity or other properties of a pesticide mixture. Surfactantsare formulants for reducing surface tension, thereby increasing the emulsifying, spreading,dispersibility or wetting properties of liquids or solids.
26GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
5.1.2 Aquatic toxicityIn the last few years, independent research on the toxicity ofglyphosate and its formations has found that they are biologicallyactive in aquatic systems at concentrations that could arise fromroutine applications. Many different aquatic organisms have beenaffected.■■
5.2 Does glyphosate affect the nervous system?Acetylcholinesterase (Ach) is an enzyme that breaks downacetylcholine, which transmits nervous impulses between nerves.Organophosphate and carbamate insecticides inhibit Ach formation,resulting in nervous impulses being maintained. Eventually, insects die- as do mammals and birds if they are exposed to high enough levelsof these pesticides. Sub-acute levels are known to cause changes inbirds and mammals in their temperature regulation, food consumptionand reproduction (Grue et al. 1997).Monsanto (1982) and some studies (e.g. Rom & Markowitz 2007)found that glyphosate has no Ach-inhibiting activity, despite thepresence of phosphate in its molecule, meaning it is not classed asan organophosphate chemical. However, other studies report thatglyphosate does suppress Ach activity in the brain, but not in muscles,of species examined (Lajmaonovich et al. 2010; Glusczak et al. 2006;Glusczak et al. 2007). The implications of these changes arising fromexposure to glyphosate have yet to be fully investigated and this needsto be carried out within a review of glyphosate herbicides.
Rotifer (Brachionuscalyciflorus)(microscopic aquatic animals)exposed to different concentrations of glyphosate had longerembryonic developmental time, longer durations of juvenile andreproductive periods, shorter average lifespan, a reduced netreproductive rate and reductions in the intrinsic populationgrowth rates (Vera et al. 2010).The parasitic horsehair worm (Chordodesnobilii)showed anumber of responses, including reduced infective capacity oflarvae and 50% mortality in adults, when exposed to glyphosateconcentrations lower than expected in freshwater environments,and lower than specified in the relevant legislation, (Achiorno etal. 2008).Phytoplankton and periphyton7communities showed changesin the microbial population structures consistent with a directtoxicological effect of glyphosate (Pérez et al. 2007).
■■
■■
5.3 Impacts on non-target plants
■■
■■
■■
As expected from its broad-spectrum use, glyphosate also impactsnon-target wild plants in field margins or in water bodies. However,A study of the combined effects of glyphosate with the trematodethere are considerable cascading effects on farmland biodiversity.parasite (Telogasteropisthorchis)and the fish parasite (GalaxiasGlyphosate, in its formulated products such as Roundup, is a highlyanomalus)found that the glyphosate and the parasite actedeffective herbicide on all types of vegetation until weed resistancesynergistically on aquatic vertebrates at environmentally-relevantdevelops (see Chapter 7), and therefore has the capacity to causeconcentrations. Researchers suggested that glyphosate mightmajor changes to the agro-ecosystem. These impacts include the lossincrease the risk of disease in fish (Kelly et al. 2010).of botanical diversity in the agro-ecosystem, including the loss of rarespecies growing in arable fields. In Iowa, US, glyphosate is classedDisturbance in the marine microbial communities was caused byas ‘high risk’ for off-target plants growing in soya and maize fields (i.e.exposure to 1 μg/l Roundup concentration, a value typical of thosethose of botanical interest but not major weeds in the crops) (Iowareported in coastal waters during run-off events (Stachowski-University State Extension 2003). Accidental drift from glyphosateHaberkorn et al. 2008).in approved use has also been found to impact on rare plants inA freshwater mussel (Lampsilissiliquoidea)was found to be acutely Australia (Matarczyk et al. 2002). Monsanto runs an ‘endangeredsensitive to Roundup and its separate components. Researchersspecies initiative’8in the US, which specifies particular areas of landtested the specific active ingredient (technical-grade isopropylamine with sensitive species present. However, this does not automatically(IPA) salt of glyphosate), the surfactant (MON 0818) and thepreclude the use of glyphosate sprayed from the air in the areacommercial product itself. MON 0818 was found to be the mostoutside the buffer zone surrounding the site.toxic, but juvenileLampsilis siliquoideawere found to be acutelysensitive to all three (Bringoff et al. 2007).Carp (Cyprinuscarpio)exhibited changes to the internalappearance of liver cells and changes to mitochondria at Roundupconcentrations between 2 and 40 times lower than used in practice(Szarek et al. 2000).
■■
It is clear that glyphosate can be toxic to many aquaticorganisms if it enters watercourses(see Chapter 4).
78
Periphyton is the complex of algae and micro-organisms attached to underwater surfaces.http://www.monsanto.com/ourcommitments/Pages/glyphosate-endangered-species-initiative.aspxGM Freeze and Greenpeace| GRL-TN 03/2011 |June 201127
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Impacts of GM herbicide-tolerant crops on weed abundance and thebiodiversity food chain were studied during the Farm Scale Evaluations(FSE) in the UK between 2000 and 2003 (Heard et al. 2003a; Heardet al. 2003b; Roy et al. 2003). The only GM RR crop trialled was beet.The other GM herbicide-tolerant crops (oilseed rape and maize) weretolerant to a different herbicide, glufosinate ammonium. Equivalentdata are not available for any GM RR crops elsewhere in the world.The conclusions were clear:
The FSE research showed how weed seed production (seed rain) wasreduced by the use of glyphosate on GM RR beet and warned that‘relatively small differences could eventually sum to produce a largeeffect if they were sustained over several crop rotations, say for 10 ormore years’ (Heard et al. 2003a).Data on GM RR maize and oilseed rape were not included in the FSEreports, as only glufosinate ammonium-tolerant GM varieties of thesecrops were trialled. In the trials, glufosinate ammonium-tolerant GM oilseed rape demonstrated similar impacts on biodiversity to GM RR beetbut the herbicide-tolerant GM maize apparently showed less adverseeffects on weed abundances than the non-GM variety, but the trial wasinvalidated by the use of a herbicide, atrazine, that was subsequentlybanned. Retrospective analysis suggested that the removal of theatrazine from the analysis very much reduced any effects on weedabundance (Perry et al. 2004).
‘Based on the evidence provided by the FSE resultspublished in October 2003, if [GM herbicide-tolerantRR] beet were to be grown and managed as in the FSEsthis would result in adverse effects on arable weedpopulations, as defined and assessed by criteria specifiedin Directive 2001/18/EC, compared with conventionallymanaged beet. The effects on arable weeds would belikely to result in adverse effects on organisms at highertrophic levels (e.g. farmland birds), compared withconventionally managed beet’(ACRE, 2004)
5.4 SummaryThere is a growing body of scientific evidence that glyphosate isharmful to species at many stages along the food chain, includingthe aquatic food chain. Scientific evidence shows that glyphosate(and its formulated commercial products such as Roundup) can haveimmediate and long-term, direct and indirect toxic effects on plantsand animals, as well as indirect effects linked to the changes it causesin the ecosystem.This new evidence of glyphosate toxicity, together with theincrease in glyphosate usage associated with GM RR crops(see Chapter 1)is now of great concern. It is time that regulatorsexamined the new evidence of harm in aquatic ecosystems thatis now emerging from independent research on toxicity andmobility in soil and aquatic systems.
� GREENPEACE / IAN CLARKE
Fig 5:Use of glyphosate-based herbicides on RR crops affectsbiodiversity.The use of glyphosate on RR beet reduces the numbers ofweeds which form the base of the food chain needed to support farmlandbirds, such as the skylark (ACRE 2004).
28GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
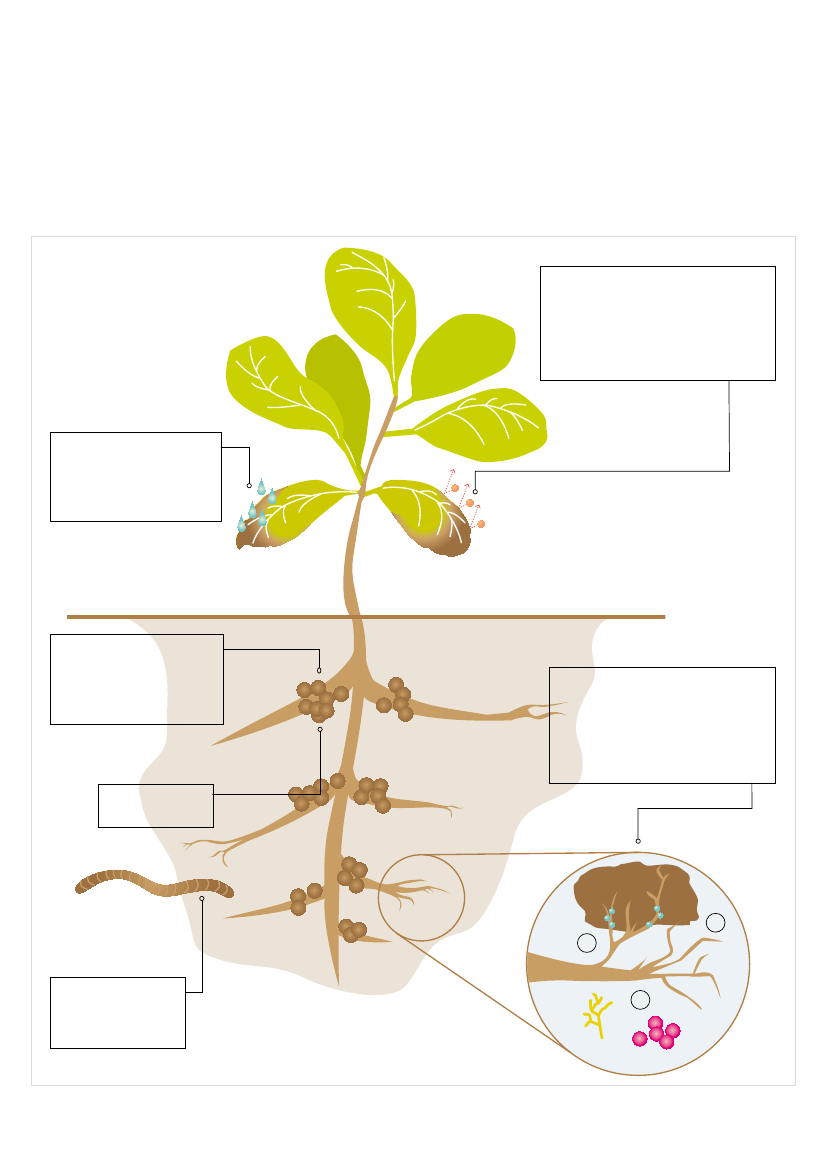
6] Glyphosate impacts on the soil-plant systemGlyphosate enters the soil by being directly sprayed on it, via theroots of plants that have been sprayed, or from dead vegetation.Glyphosate is soluble in water and can be washed into the soil byrainfall or irrigation. In some soils, it can bind tightly to soil particles.This means it cannot be washed deeper into soil and is less likely to bedegraded by soil microbes. In other soil types, it remains mobile in soilwater and can be leached into drains and decomposed. Tightly-boundglyphosate can be displaced by other chemicals, such as phosphate,meaning it can become present in soil water again.The interactions between the chemical, physical and biologicalcomponents of the soil and glyphosate are complex (Kremer &Means 2009; Zablowicz & Reddy 2004). Given this complexity, therequirements for the risk assessment of glyphosate by regulatorsaround the world are surprisingly limited. For instance, in the EU,applicants are only required to provide data on the persistence ofglyphosate in the soil and the impact on earthworms and otherfunctional groups of soil organisms. Detailed examination of the impactof glyphosate on the make-up and activity of soil microbial speciesincluding pathogens is not required (EU Commission, DirectorateGeneral for Health – DG SANCO 2002).Glyphosate binds so tightly to soil particles that it is rendered inactiveand hence unavailable to organisms. It is therefore claimed thatglyphosate has limited biological availability in the soil (Monsanto2005b; Geisy et al. 2000). However, several important interactionsbetween glyphosate and soil microbes have been identified thatimpact on the function of plants (Kremer & Means 2009). Theseinteractions are detailed in Fig. 6.When glyphosate is available in the soil, it affects microbialcommunities leading to:■■■■■■■■
6.1 Availability of glyphosate in the soilThe impact that glyphosate will have on the soil ecosystem is largelydependent on whether it is bound to soil particles or unbound andfree. Glyphosate molecules bind with particles present in the soil thathave a high binding capacity - such as aluminium hydroxide and ferricoxides, minerals or organic matter - and subsequently become inert(Shushkova et al. 2009). The extent to which this happens varies fromsoil to soil, depending on its composition and on the presence of otherchemicals and mineral nutrients. When glyphosate is not bound (oronly loosely bound) to soil particles, it is available for microbes to breakdown and utilise as a source of energy and nutrients. It is when thishappens that glyphosate starts to impact on the environment.Soil chemistry can also play an important part in how much or how littleglyphosate binds to the soil. Phosphate competes with glyphosatemolecules for soil-binding sites. In experiments, soils to which aphosphate solution has been applied are found to have raised levelsof glyphosate and AMPA in solution, thus making it mobile in the soil(Simonsen et al. 2008) and available for microbes to break down orleach through the soil.The rhizosphere is the thin layer of soil immediately surrounding plantroots, an area that is extremely important for the uptake of nutrientsinto the plant. It is markedly different from the bulk soil (Chin-hua &Palada 2006; see Fig 6). Glyphosate appears to interfere with thebiological and chemical processes in this important rhizosphere,unintentionally affecting plant growth and nutrition.
6.2 Activity and abundance of soil microbesMolecules of glyphosate and its breakdown product AMPA, whichare not bound to soil particles, are available for soil and rhizospheremicrobes (also called micro-organisms) to utilise as a source ofnutrients and energy, thus leading to increased microbial biomass andactivity (Haney et al. 2000; Wardle & Parkinson 1990). Glyphosate cantherefore affect the structure of the microbial community, increasingthe abundance of some microbes and decreasing others.The existence of the EPSPS enzyme in microbes and fungi meansthey can be affected by the presence of free glyphosate in the soil, andchanges in microbial populations occur in the rhizosphere of GM RRcrops depending on how susceptible they are to glyphosate. Somegroups increase, such as manganese-oxidising bacteria, and somedecrease, such as pseudomonads that act against fungal pathogens.Thus, important roles for microbes such as growth promotion andbiological control can be disrupted (Kuklinsky-Sobral et al. 2005).
Reduction in mineral uptake by crops;Increased microbial biomass and activity;Proliferation of phytopathogens in crops;Reduction in nitrogen fixation and nodulation, leading to increaseddemands for nitrogen fertiliser.
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201129
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Application of glyphosate reduces plantuptake of the essential micronutrientmanganese (Mn), requiring additionalapplications of Mn to GM Roundup Readysoybeans. Uptake of another essentialmicronutrient, Zn, is also reduced.
Application of glyphosateincrease susceptability toinfection by harmful bacteriaand fungi, e.g. Fusarium,take-all and root rot.
NITROGEN (N) Glyphosateaffects N - fixing bacteriaassociated with root nodules.N is a major plant nutrient sothis results in lower yield.
Glyphosate (Gly) enters the soil andundergoes complex interactions withsoil particles and microbes (bacteriaand fungi) in the soil-root zone. This isof importance as this region particularlyaffects plant growth and health.
N-fixing bacterialroot nodules.
THERHIZOSPHERE
Soil mineral/organic particlesGlyGly
Glyphosate mayadversely affectearthworms, althoughresearch is limited.
Fungi
GlyBacteria
Fig 6:Plant-soil interactions of glyphosate.30GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
A recent study on Monsanto’s new variety of GM RR soya (RR2)found that glyphosate negatively impacted ‘the complex interactionsof microbial groups, biochemical activity and root growth that canhave subsequent detrimental effects on plant growth and productivity’(Zobile et al. 2011b). The plant roots had higher numbers ofFusariumspecies and reduced numbers of manganese-reducing bacteria.These imbalances in the soil and rhizosphere microbial community canaffect soil and plant health. They may reduce the availability of plantnutrients, or cause increased vulnerability to disease.
6.3 Reduced nutrient uptake by plantsResearch is beginning to uncover the complex relationship betweenglyphosate use and plant nutrients, such as manganese and zinc.Manganese is vital to plants, enabling photosynthesis, enzymefunction, nitrate assimilation and the formation of vitamins (Ducic& Polle 2005). Zinc is vital in the structure and function of enzymes(Broadly et al. 2007).Glyphosate has been shown to interact with micronutrients, such asmanganese, in the rhizosphere and appears to reduce their availabiltyto plants. These interactions possibly happen because glyphosateaffects micro-organisms in the rhizosphere (see Chapter 5). Laboratoryexperiments with sunflowers show that after glyphosate applicationthere was ‘an impairment of the manganese-nutritional status, whichwas still detectable after a waiting time of up to 21 days’ (Tesfamariamet al. 2009). This confirmed previous field observations and researchsuggesting that glyphosate affected microbes associated with themobilisation of rhizosphere manganese. This then influences themobility of manganese in the rhizosphere and hence reduces theamount that is available to the plant even when the element is plentifulin the soil (Kremer & Means 2009). Applications of manganese to GMRR soya are necessary to counteract this reduction in manganeseuptake compared with the conventional varieties (Gordon 2007).Studies on GM RR soya confirm these findings and suggest thatother nutrients may also be affected by a reduced availability to theplant. Research in Brazil showed that the application of glyphosate‘interfered [with the] mineral nutrition of soybean and the total contentsof [nitrogen, manganese, copper, zinc and iron]’ (Serra et al. 2011).Recent research on Monsanto’s GM RR soya 2 has shown that italso suffers from reduced macro-and micro-nutrient as a result ofglyphosate applications (Zobiole et al. 2011a).
One study found that ‘nodulation was always lower on GR [GMglyphosate-resistant] soybean with or without glyphosate comparedwith conventional varieties with non-glyphosate or no herbicide’(Kremer & Means 2009). The authors suggested that ‘glyphosate andperhaps genetic modification in the GR plant may affect the numerousprocesses involved with nitrogen fixation symbiosis’, which processesinclude nitrogenase activity and leghaemoglobin (an oxygen-carryingprotein found in plants) content. This effect could have a significantimpact on the long-term sustainability of the crop, which relieson fixation to provide 40% to 70% of the nitrates required by thecrop (Kremer & Means 2009). In the longer term, this will lead to anincreased dependency on the external addition of nitrates (Bohm et al.2009), along with the all the environmental concerns associated withthe use of nitrate fertilisers (Tillman 1999).It is clear that GM RR crops, especially RR soya, suffer from a range ofnutrient deficiencies induced by the application of glyphosate.6.3.2. Glyphosate toxicity to earthwormsIndependent research on the impact of glyphosate in earthwormsis limited. However, there is evidence that repeated use candamage this vitally important group of species. Growth rates of oneearthworm species were reduced in culture chambers for 100 daysby a range of glyphosate concentrations (Springett & Gray 1992).Similar findings were found in a different species (Yasmin & D’Souza,2007). Another study found a significant change in the hatching ofearthworm cocoons exposed to glyphosate and that the earthwormsactively avoided glyphosate-treated soil in the laboratory (Casabéet al. 2007). The conclusion was that the study ‘showed deleteriouseffects of [glyphosate] … formulations when applied at the nominalconcentrations recommended for soya crops’.The repeated use of glyphosate on GM crops and in general weedcontrol across a wide range of agro and forest ecosystems couldpossibly have long-term impacts on earthworm populations. Given thevital importance of earthworms in improving soils and in the food chain,there is an urgent need to assess the impact of long-term exposure.
6.4 Increased vulnerability to plant diseases
‘The synergistic activity of glyphosate weed control inpredisposing plants to infectious organisms has beenobserved for many diseases … and the extensive use6.3.1 Nitrogen fixation impairedIt is known that nitrogen fixation and uptake is affected in GM RR soya.of glyphosate in agriculture is a significant factor in theConventional agriculture normally relies upon the addition of a nitrogenincreased severity or ‘re-emergence’ of diseases oncefertiliser to the soil. However, some crops - such as legumes (peas,considered efficiently managed’beans etc.) - are able to utilise atmospheric nitrogen. They do this byforming symbiotic relationships with ‘nitrogen-fixing’ bacteria in thesoil. The bacteria form characteristic nodules around the roots of theplants. There is evidence that the functioning of these nodules in GMsoya plants is affected by the presence of glyphosate.(Johal & Huber 2009)
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201131
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
The impact of glyphosate on the ability of plants to fight off disease hasnow been extensively studied. Pathogenic (or harmful) fungal diseaseshave been found to worsen in the presence of glyphosate in 13 crops,including soybeans, wheat, cotton, sugar beet and canola. Diseasesinclude take-all in cereals, damping off, root rot and sudden deathsyndrome in soybeans (Johal & Huber 2009).Several plant diseases caused byFusariumspecies (pathogenic fungi)are increased by glyphosate. The presence of the herbicide in therhizosphere enables these fungi to become the dominant group (Kremer& Means 2009). Soil-dwellingFusariumspecies cause a wide range ofdiseases in crop plants, ornamentals and grasses (Nelson et al. 1983).Therefore, any increase arising from the use of glyphosate could besignificant in a wide range of different land uses, including GM RR crops.Research on the impact of glyphosate on microbes inhabiting soil outsidethe rhizosphere is limited (Kremer & Means 2009). Other research foundthat numbers of the soil-borne pathogen (or disease)Pythium ultimumwere significantly greater in root exudates from bean plants treated withglyphosate than those that were not (Kuklinsky-Sobral et al. 2005). OtherPythiumspecies were not stimulated in this way.
6.5 Case studies of glyphosate impacts onsoil-plant system6.5.1 Root rot in soybeansThe roots of soybean plants growing near giant ragweed plants(Ambrosiatrifida)that had been treated with glyphosate in Indiana, US,were found to have black lesions on 90 to 95% of their roots, causedby the pathogenic fungusCorynespora cassiicola.By comparison,lesions were found on only 5-10% from plants growing next to non-treated ragweed. Yield losses from plants affected by glyphosatesprayed weeds were four times those of other soybean plants(Huber et al. 2005).6.5.2 Citrus variegated chlorosis (CVC)
The symptoms of this disease are a yellowing of the leaves of citrusplants, similar to that caused by manganese and zinc deficiencies.Weed control in citrus plantations in Brazil involved the use ofglyphosate. The method developed by the International Plant NutritionInstitute in Brazil was to stop using glyphosate for weed control aroundthe trees and replace it with a mulch of grass cut from between theThe toxicity of glyphosate to soil microbes is highly variable and is thought rows (Yamada & Castro 2005). This controlled weeds and restoredto be dependent on the sensitivity of the microbe’s EPSPS enzyme tomanganese and zinc to sufficient levels in the soil. The removal ofglyphosate. For instance, five species of the ubiquitous family of soilglyphosate also reduced the incidence of crown rot (PhytophorabacteriaPseudomonaswere found not to be sensitive to glyphosate,species).while the activity of one commonly-found member of the family,Pseudomonas fluorescens,was inhibited by glyphosate (Schullz, Krüper6.5.3 Take-all in cereals& Amrhein 1985).Pseudomonasspecies are thought to be importantThe take-all fungus,Gaeumannomyces graminis(ascomycota), isin the breakdown of glyphosate in the soil (Gimsing et al. 2004). Othera major root-rot pathogen of cereals and grasses. Glyphosate ismicrobes use glyphosate and AMPA as a source of phosphorus.used extensively to clear weeds before cereals are sown, and it hasThere are a number of possible explanations why GM RR soyatreated with glyphosate should suffer from increasedFusariumactivitycompared with crops not sprayed with glyphosate (Powell & Swanton2008):■■
been recognised for many years that take-all infection is increasedfollowing the use of the herbicide (Johal & Huber 2009). Take-all isalso increased following applications of glyphosate associated withthe cultivation of GM RR soya the preceding year in comparison withthe use of other herbicides. It is thought that this increase in take allStimulation of pathogenic fungi by glyphosate, either via the roots or severity is thought to be associated with Mn-deficiency induced byby direct application to the soil.glyphosate (Johal & Huber 2009).Glyphosate may inhibit of the production of antagonistic chemicalsproduced by the plant to protect itself (for example, Phytoalexinsare produced by plants to protect them from harmful microbes).Glyphosate may inhibit the production of protective plant tissuessuch as lignin.Increased levels of pathogen inoculum on weeds if control in theGM RR crop is delayed.Enhanced stimulation of pathogens compared with microbes thatare antagonistic to pathogens.
■■
6.6 SummaryWhen sprayed, glyphosate enters the soil either directly or indirectly.In the soil, the inter-relationships between glyphosate, AMPA, soilminerals and microbes are complex.It is not known exactly how glyphosate influences soil processes, orsoil health. However,it is now clear that presence of glyphosatein the rhizosphere can affect the uptake of essentialmicronutrients from the soil, the fixing of nitrogen by rootnodules and increase plant’s vulnerability to diseases.
■■
■■
■■
32GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
7] Weed resistance and glyphosate - the failure ofGM Roundup Ready technologyAs with GM crops in general, GM herbicide-tolerant crops are primarilygrown in North America, Brazil and Argentina (ISAAA 2011; see Chapter1). Farmers who adopted these GM crops did so because the seedswere advertised (e.g. Monsanto 2009a) as allowing better and easierweed control that would make crop management much simpler,cheaper and less time intensive (see Chapter 1). Consequently, GM RRsoya, maize and cotton have gained significant market shares in theAmericas.When they first appeared in the mid 1990s, weed resistance toherbicides as a result of GM RR crops was rarely discussed, althoughthe phenomenon of weed resistance to herbicides was well known.Now, 15 years later, weed resistance to glyphosate is one of the mostwell documented effects and a ‘major environmental concern’ of thecultivation of GM RR crops (IAASTD 2009a).A survey of 1,200 farmers across six US States in 2005/06 found thatonly 3 out of 10 farmers thought that glyphosate-resistant weeds werea serious issue and a ‘substantial number of farmers underestimatedthe potential for glyphosate resistant weed (GR) populations to evolvein an agro-ecosystem dominated by glyphosate as the weed controltactic’ (Johnson et al. 2009). Other studies have confirmed thatfarmers do not start to manage for weed resistance until they haveappeared in their fields (Beckie 2006):
7.1 A false dawnFor the first few years following their introduction in 1996, GM RRcrops did indeed perform as the adverts had promised. Roundupwas widely, and possibly indiscriminately, used on GM RR crops,as evidenced by the increase in glyphosate usage (see Chapter 1).Farmers soon became dependent on glyphosate as their main meansof chemical weed control in fields of GM RR soya, maize and cotton.They were unaware - or possibly unconcerned - that weed resistancecould occur on their farms. However, it was only a very short timebefore the first glyphosate resistant weed - horseweed (Conyzacanadensis)- was confirmed in areas of soybean cultivation in 2000(Zelaya et al. 2004).
‘Part of the reason growers do not manage proactively isbecause most know other options are still available andexpect companies to continue to provide new technology.Unfortunately, companies are not being as successful indiscovering selective herbicides with new modes of actionas they have been in the past.’(Green 2008)
In Argentina, the first glyphosate resistant weed to be recorded wasJohnsongrass (Sorghum halepense) in 2002 (Binimelis et al. 2009).However, early warnings were not heeded, and farmers who raisedconcerns in the Las Lijatas (northern Argentina) area were reassured.
‘Little attention was paid to the uncontrolled clumps[of glyphosate-resistant Johnsongrass] and the farmerUS scientists have suggested that the rapid spread of glyphosatecomplained that no effective action was taken becauseresistance in horseweed can be traced to the use of zero tillage inthey were misled by technical advisors who stated thatGM RR crops. Zero tillage is where the soil is not ploughed or tilled. Itthe situation was not problematic. This year, upset by theused as a soil and carbon conservation tool by both sustainable andincreasing severity of the resistance problem, he decidedindustrial agricultural systems. In industrial agricultural systems, it isoften associated with GM herbicide-tolerant crops where weed controlto speak up and talk to the local press to raise awarenessand speed up proper action.’is achieved by the use of herbicides.(Valverde & Gressel 2006)
‘No-tillage corn (Zea mays L.) and soybean (Glycinemax (L.) Merr.) production has been widely acceptedin the mid-Atlantic region, favouring establishment ofhorseweed (Conyza canadensis (L.) Cronq). Within 3 yearsof using only glyphosate for weed control in continuousglyphosate-resistant soybeans, glyphosate failed to controlhorseweed in some fields. Seedlings originating fromseed of one population collected in Delaware were grownin the greenhouse and exhibited 8 to 13-fold glyphosateresistance compared with a susceptible population.’(Van Gessel 2001)
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201133
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
This lack of attention to the early signs of weed resistance may havebeen important in deepening the problems of weed resistance inArgentina. Johnsongrass is ‘one of the world’s worst weeds’(Bryson 2000) and hence there is real concern about its suddenresurgence in GM RR soya.
now been confirmed in over 20 species and over 100 resistantstrains have been identified (Table 2). Although these weeds occur inseveral continents, they primarily occur in the US and the Americas(Fig. 7), where GM RR crops are primarily grown (see Chapter 1).Weed scientists link this rapid growth to the expansion of GM RRcrops and there is widespread concern about the agricultural andeconomic impacts this has and will be causing.‘
‘…The evolution of glyphosate-resistance in S. halepense[Johnsongrass] is a major threat to glyphosate-resistantsoybean productivity in northern fields of Argentina.’(Vila Aiud et al. 2008)
A glyphosate-resistant biotype (variety or strain) of Johnsongrasshas also been found in Arkansas in 2005, once again in areas ofGM RR soya cultivation (International Survey of Herbicide ResistantWeeds 2010).The first reported case was confirmed in Rigid Ryegrass (Loliumrigidia)in Victoria, Australia in 1997 (Powles 1998). Since then the numberof species and strains with glyphosate resistance has increaseddramatically (Fig. 7). From zero in 1996, glyphosate resistance has120
‘Because glyphosate is the herbicide most often usedin no-till and minimum-till systems, GR [glyphosateresistant] volunteer crop plants and glyphosate-resistantor tolerant weeds will jeopardise the sustainability ofthose systems.’(Mallory-Smith& Zapoila 2008)
No. of resistant strains recorded
100
80
60
40
20
01996199820002002200420062008
2010
Year
Global cumulative total
US cumulative total
Americas excl. US cumulative total
Fig 7:Increase in the occurrence of glyphosate resistant weeds over the past 15 years in the US, the Americas and globally.GM Roundup Ready (RR)crops (resistant to glyphosate) were first introduced in the mid 1990s and grown principally in the US and elsewhere in the Americas (South America and Canada).The occurrence of glyphosate-resistant weed strains follows the pattern of GM RR cultivation with the highest frequency occurring in the Americas, notably the US,and increasing in the years since the introduction of GM RR crops. Based on data from the International Survey of herbicide Resistant Weeds www.weedscience.org.
34GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Table 2:Infestation levels of glyphosate-resistant weed species around the world9Glyphosate-resistantweedCountryNumber oflocations whereresistanceconfirmed101177121Estimatedmaximum totalarea infested (alllocations) (ha)2 030 000413 0004 9609 7804.058 62020.23 340 00081 4006072082028 68010 400Crops affectedYearglyphosateresistancefirst detected20052005200420042010
Palmer amaranthAmaranthus palmeriCommon water hempAmaranthus rudisCommon ragweedAmbrosia artemisiifoliaGiant ragweedAmbrosia trifidaAustralian FingergrassChloris truncataHairy FleabaneConyza bonariensisSumatran fleabainConza sumatrensisHorseweed/MarestailConyza canadensisSourgrassDigitaria insularisJunglericeEchinochloa colonaGoosegrassEleusine indicaWild poinsettiaEuphorbia heterophyllaItalian ryegrassLolium multifloriumRigid ryegrassLolium rigidiaPerennial ryegrassLolium perennesRagweed PartheniumParthenium hysterophorusKochiaKochia scopariaBuckhorn PlantainPlantago lanceolataJohnsongrassSorghum halepenseLiverseedgrassUrochloa panicoidesBlue grassPoa annua
USUSUSUS, CanadaAustralia
Maize, cotton and soyaMaize and soyaSoyaSoya, cotton and maizeCropland
US, Colombia, Brazil, Spain, 9South Africa, Israel, AustraliaSpain1US, Brazil, Spain, China,Czech RepParaguay, BrazilAustraliaColombia, MalaysiaBrazilChile, Brazil, US,Spain, ArgentinaItaly, Spain, France, SouthAfrica, Australia, USArgentinaColombiaUSSouth AfricaUS, ArgentinaAustraliaUS2352311114
Roadsides, fruit and orchards, maize, 2003wheat, soyaOrchards2009Soya, maize, cotton, rice, fruit,orchards, road side, railway, nurserySoya, orchardsCroplandCropland, coffee, cottonSoyaCropland, orchards, fruit, cotton,soya,barley, wheatOrchard and vineyards, asparagus,cereals, wheat, almonds, cropland,sorghumBarley, cropland, soya, wheatFruitMaize, cotton, cropland and soyaOrchards and vineyardsSoyaSorghum, wheatTurf2000200620071997200620011996
1121411
20.240.44 05020.240 50020.24.045 960 000
2008200420072003200520082010
Total global area glypohosate resistant weeds (2010)
9Data from the International Survey of Herbicide Resistant Weeds (www.weedscience.org). It does not include glyphosate resistant volunteer crops, which are also common in RR crops.10Represents the number of locations where the resistant biotypes are found. The number of infestations at each location range from one to 100 000.GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201135
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
The confirmation of glyphosate resistance in more species in the nearfuture seems highly probable. Twenty-one other weeds in Argentinahave been listed as ‘just barely controlled by glyphosate’ and ‘mightbe the next to upgrade to full resistance by another evolutionary step’(Valverde & Gressel 2006). These include field bindweed (Convolvulusarvensis),curled dock (Rumexcrispus)and morning glory (Impomoeapurpurea).It is very clear that weed resistance to glyphosate is an increasingproblem across the globe and most prominently where GM RR cropsare widely grown. The ramifications of this increased weed resistanceare that extreme measures are recommended by Monsanto to prolongthe effectiveness of GM RR crops.
Monsanto has published guidance on how to deal with the growingweed resistance problems in GM RR crops (Monsanto 2011) and hasalready begun developing prevention strategies based on the use ofcombinations of herbicides and timing of applications.■■
The first strategy isthe use of either stronger formulations ofglyphosate11or of tank mixtures of glyphosate and otherherbicides.For instance, 2,4-D – one of the active ingredientsof Agent Orange, the defoliant used by the US Army during theVietnam War – is recommended for burning down weeds prior tosowing marestail (Monsanto 2008).The second strategy isto produce seeds with several herbicidetolerant genes (gene stacking) by crossing different GMherbicide-tolerant varietiesso that different herbicides can beapplied to the growing crop in rotation or in tank mixes. This willensure that weeds that are resistant to glyphosate will be killed byother herbicides (see, for example, Monsanto 2009). For instance,Monsanto has recently announced an agreement with the chemicaland biotechnology company BASF to develop crops stacked withglyphosate and dicamba tolerant genes (Monsanto 2010c; seealso Behrens et al. 2007; Service 2007). Less is known about thetoxicity of dicamba but there are concerns it may be toxic to aquaticorganisms12.The third strategy isto use herbicides that remain active in thesoil (residual herbicides or residuals),which kill seedling weedsas soon as they germinate.
■■
‘…More worrisome are glyphosate-resistant populationsof far more economically damaging weed species [thanLolium].’(Powles 2008)
7.2 Monsanto’s Reaction to reports of resistantweedsMonsanto is highly aware of the growing resistance problem in GMRR crops but appears reluctant to take on liability for the extra costscurrently being borne by farmers.■■
‘Growers must be aware of and proactively managefor glyphosate-resistant weeds in planning their weedcontrol program. When a weed is known to be resistantto glyphosate, then a resistant population of that weedis by definition no longer controlled with labelled rates ofglyphosate. Roundup agricultural herbicide warrantieswill not cover the failure to control glyphosate-resistantweed populations.’(Monsanto, undated)
It is clear from these strategies that weed resistance is a seriousproblem for the continued efficiency of GM RR crops, and tacklingthe problem requires extreme measures. Currently, no cost estimatesare available for the increased amount of farmer expenditure onweed control as a result of weed resistance, but they are likely to beconsiderable. These extreme measures may have environmental andpossibly health implications.
‘Most of the documented cases of evolved GR[glyphosate resistant] weeds in the past six years havebeen in GR crops.’(Duke and Powles 2008)
1112
For example, ‘Roundup Weather MAX’. See http://www.monsanto.com/products/Pages/roundup-weathermax-herbicide.aspxPesticide Action Network. See http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC32871
36GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
7.3 False solutions create more problemsThe strategy of applying tank mixes pre and post-emergence andresidual herbicides will add to the amounts being used and to theoverall toxic burden on the environment from GM RR crops.This strategy could also create new problems if herbicides areoverused, leading to the evolution of weeds with resistance to severalherbicides. The problem of multiple weed resistance is already wellestablished in the US soya and corn belts. Several weeds that havedeveloped glyphosate resistance are also resistant to other herbicides.In soya crops one strain of common waterhemp (Amaranthusrudis)already has resistance to sulphonylureas (e.g. thifensulfuron-methyl),triazines (e.g. atrazine) and glyphosate. Another has resistance tosulphonylureas (e.g. cloransulam-methyl), triazines (e.g. atrazine) anddiphenyl ethers (e.g. Lactofen). Twelve other strains have double-resistance. In all there are already seven biotypes in soya crops withmultiple resistances that include a resistance to glyphosate. In maize,there are three multi-resistant biotypes including glyphosate. Many ofthese problem weeds are common to both crops (International Surveyof Herbicide Resistant Weeds 2010).Options for using tank mixes of herbicides or soil residuals to cope withweed resistance are already limited in both crops by the numerous(over 20) weed species that already have resistance to one or moreactive ingredients.The future strategy for dealing with glyphosate-resistant weedsinvolves the use of a wide range of active ingredients in mixtures orsingly with a cocktail of adjuvants used in each formulated product.The toxicology of such mixtures is unclear, as approval processestend to focus on the individual products rather than any additive and/or synergistic effects. Nor can the development of more weeds withmultiple resistances in the future be ruled out. Sustainable solutionswill not come from the continual adherence to the crop monoculturesreliant on chemical weed control that GM RR crops epitomise.
7.4 ConclusionThe rapid evolution of weeds that are resistant to glyphosate is a resultof farmers becoming over-reliant on one herbicide for weed control.This is particularly associated with GM RR crops. Now that resistanceto glyphosate is widespread in weeds within GM RR soy, maize andcotton crops, farmers have to resort to using mixtures of herbicides.Thus, the promise of reduced herbicide use and cheaper andeasier weed controls has not been delivered. However, it isclear that GM RR crops have brought about an escalation in thepesticides ‘arms race’, with an increasing toxic burden on theenvironment involving significant uncertainty about the overallsafety of glyphosate for people and biodiversity.
GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201137
� GREENPEACE / RODRIGO BALÉIA
Farmers are having to resortto stronger formulationsof glyphosate or herbicidemixtures to cope with weedsresistant to glyphosate. Thishas resulted in an escalationof the pesticides ‘arms race’,with an increasing toxicburden on the environment.
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
8] ConclusionsThere are many problems with GM crops, fundamentally theirtendency to produce unexpected and unpredictable effects. This isrelated to the process by which they are created, the forcible insertionof gene(s) into a plant genome. No GM crops should be cultivated inthe environment, nor enter the food chain.In this report, the focus is on one GM trait, that of herbicide resistance- in particular, it looks at the close association between RoundupReady crops and the herbicide glyphosate. The evidence presentedin the report demonstrates that glyphosate-based herbicides, suchas Roundup, can have harmful effects on human health and theenvironment.Exposure of humans to glyphosate has been linked to various healtheffects, including reproductive effects, cancer and neurologicaleffects. Glyphosate interacts with soil chemistry and biology, resultingin a variety of impacts including reduced plant nutrition and increasevulnerability to plant diseases. Glyphosate may also leach into surfaceand groundwater, where it may damage wildlife and possibly end up indrinking water.Thus, glyphosate and Roundup are far from benign herbicides.A review of their safety for human and animal health and for theenvironment is urgently needed.GM RR crops have greatly increased glyphosate usage, especially inthe Americas where they are primarily grown. Given the new evidenceof glyphosate toxicity, this is of great concern. The rise in glyphosate-resistant weeds is associated with GM RR crops, and the escalation inthe ‘arms-race’ against these resistant weeds fuels concerns that evenmore glyphosate, in stronger formulations and possibly with additionalherbicides, will be used on GM RR crops in the future. Similarproblems would be highly likely to arise if other GM herbicide-resistantcrops are widely cultivated and farmers become dependent on a singleherbicide, e.g. GM crops resistant to glufosinate ammonium, marketedas ‘Liberty Link’. This facet of GM herbicide-tolerant crops should beenough to lead to a ban on their cultivation.GM herbicide-tolerant crops, as epitomised by GM RR crops, are notpart of a sustainable agriculture system. As with all GM crops theyhave been developed for, and are economically profitable within, anindustrial agriculture system that involves large-scale monoculturesthat depend on costly, polluting inputs such as herbicides, syntheticfertilisers and fossil fuels.In contrast, ecological farming both relies on and protects nature bytaking advantage of nature’s goods and services, such as biodiversity,nutrient cycling, soil regeneration and natural enemies of pests, and byintegrating these natural goods into agro-ecological systems.The widespread and increasingly intensive use of glyphosatein association with the use of GM RR crops, poses risks to theenvironment and human health. Given the problems that arenow evident, no new GM glyphosate-tolerant crops shouldbe authorised. As part of broader considerations of the wayforward for agriculture, no GM herbicide-tolerant crop can formpart of a sustainable agriculture model, and the cultivation of allsuch crops should be banned.
GM herbicide-tolerant crops, as epitomised by GMRR crops, are not part of a sustainable agriculturesystem…In contrast, modern agro-ecological farmingpractices both relies on and protects nature by takingadvantage of nature’s goods and services.
38GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
ReferencesAchiorno CL, Villalobos C & Ferrari L. 2008. Toxicity of the herbicide glyphosate to Chordodesnobilii (Gordiida, Nematomorpha). Chemosphere 71: 1816-22.CRE. 2004. Advice on the implications of the farm-scale evaluations of genetically modifiedherbicide tolerant crops. http://webarchive.nationalarchives.gov.uk/20080727101330/http://www.defra.gov.uk/environment/acre/advice/pdf/acre_advice44.pdfAhsan N, Lee DG, Lee KW, Alam I, Lee SH, Bahk JD & Lee BH. 2008. Glyphosate-inducedoxidative stress in rice leaves revealed by proteomic approach. Plant Physiology Biochemistry46: 1062-70.Anadón A, Del Pino J, Martínez MA, Caballero V, Ares I, Nieto I & Martínez-Larrañaga MR. 2008.Neurotoxicological effects of the herbicide glyphosate. Toxicological Letters 180S: S164.Arbuckle TE, Lin Z & Mery LS. 2001. An exploratory analysis of the effect of pesticide exposureon the risk of spontaneous abortion in an Ontario farm population. Environmental HealthPerspectives 109: 851-57.Astiz M, De Alaniz MJT & Marra CA. 2009. Effect of pesticides on cell survival in liver and brainrat tissues. Ecotoxicology Environmental Safety 72: 2025-32.Axelrad JC, Howard CV & McLean WG. 2003. The effects of acute pesticide exposure onneuroblastoma cells chronically exposed to diazinon. Toxicology 185: 67-78.Barbosa ER, Leiros da Costa MD, Bacheschi LA, Scaff M & Leite CC. 2001. Parkinsonism afterglycine-derivative exposure. Movement Disorders 16: 565-8.Battaglin WA, Kolpin DW, Scribner EA, Kuivila KM & Sandstrom MW. 2005. Glyphosate, otherherbicides, and transformation products In Midwestern Streams, 2002. Journal of the AmericanWater Resources Association 41: 323-332.Battaglin WA, Rice KC, Focazio MF, Salmons S & Barry RX. 2008. The occurrence ofglyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington,DC, Maryland, Iowa, and Wyoming, 2005-2006. Environmental Monitoring and Assessment155: 281-307.Beckie HJ. 2006. Herbicide–resistant weeds; management tactics and practices. WeedTechnology 20: 793-814.Bellé R, Le Bouffant R, Morales J, Cosson B, Cormier P & Mulner-Lorillon O. 2007. Seaurchin embryo, DNA-damaged cell cycle checkpoint and the mechanisms initiating cancerdevelopment (Translated title). Journal de la Société de Biologie 201:317-27.Behrens MR, Mutlu N, Chakraborty S, Dumitru R, Jiang WZ, LaVallee BJ, Herman PL, ClementeTE & Weeks DP. 2007. Dicamba resistance: enlarging and preserving biotechnology-basedweed management strategies. Science 316: 1185-1186.Benachour N & Séralini GE. 2009. Glyphosate formulations induce apoptosis and necrosis inhuman umbilical, embryonic, and placental cells. Chemical Research in Toxicology 22: 97-105.Benachour N, Siphatur H, Moslemi S, Gasnier C, Travert C & Séralini GE. 2007. Time- anddose-dependent effects of Roundup on human embryonic and placental cells. Archives ofEnvironmental Contamination and Toxicology 53: 126-33.Benbrook C. 2001. Troubled Times Amid Commercial Success: glyphosate efficacy is slippingand unstable transgene expression erodes plant defenses and yields, Ag BioTech InfoNetTechnical Paper Number 4, accessible at http://www.biotech-info.net/troubledtimes.htmlBenbrook CM. 2004. Impacts of genetically engineered crops on pesticide use in the UnitedStates: the first eight years. AgBioTech InfoNet Technical Paper Number 7 http://www.organic-center.org/science.latest.php?action=view&report_id=158Benbrook C. 2009. Impacts of genetically engineered crops on pesticide use in the UnitedStates: the first thirteen years. The Organic Center February 2011 http://www.organic-center.org/science.pest.php?action=view&report_id=159Beuret CJ, Zirulnik F & Giménez MS. 2005. Effect of the herbicide glyphosate on liverlipoperoxidation in pregnant rats and their fetuses. Reproductive Toxicology 19: 501-4.Binimelis R, Pengue W & Monterroso I. 2009. Transgenic treadmill: Responses to theemergence and spread of glyphosate-resistant Johnsongrass in Argentina. Geoforum 40: 623-633.Bohm GMB, Alves BJR, Urquiaga S, Boddey RM, Xavier GR, Hax F & Rombaldi CV. 2009.Glyphosate and imazethapyr induced effects on yield, nodule mass and biological nitrogenfixation in field-grown glyphosate-resistant soybean. Soil Biology and Biochemistry 41: 420-422.Bolognesi C, Bonatti S, Degan P, Gallerani E, Peluso M, Rabboni R, Roggieri P & AbbondandoloA. 1997. Genotoxic activity of glyphosate and its technical formulation Roundup. Journal ofAgricultural and Food Chemistry 45: 1957-62.Bonny S. 2008. Genetically modified glyphosate-tolerant soybean in the USA: adoption factors,impacts and prospects. A review. Agronomy for Sustainable Development 28: 21–32.Borggaard OK & Gimsing AL. 2008. Fate of glyphosate in soil and the possibility of leaching toground and surface waters: a review. Pesticide Management Science 64: 441-456.Botta F, Lavison G, Couturier G, Alliot F, Moreau-Guigon E, Fauchon N, Guery B, Chevreuil M &Blanchoud H. 2009. Transfer of glyphosate and its degradate AMPA to surface waters throughurban sewerage systems. Chemosphere 77: 133-139.Bradberry SM, Proudfoot AT & Vale JA. 2004. Glyphosate poisoning. Toxicological Review 23:159-67.Bringolf RB, Cope WG, Mosher S, Barnhart MC & Shea D. 2007. Acute and chronic toxicityof glyphosate compounds to glochidia and juveniles of Lampsilis siliquoidea (Unionidae).Environmental Toxicology and Chemistry 26: 2094-2100.Broadley MR, White PJ, Hammond JP, Zelko I & Lux A. 2007. Tansley Review Zinc in Plants.New Phytologist 173: 677–702.Bryson C. 2000. History and future importance of Sorghum halepense (L.) Pers. in the USA.Abstracts of the Third International Weed Science Conference. Brazil 2000.Canadian Council for Environment Ministers. 1999. Canadian Water Quality Guidelines for theProtection of Aquatic Life.Canadian Food Inspection Agency. 2005-2006. National Chemical Residue MonitoringProgram Annual Report. http://www.inspection.gc.ca/english/fssa/microchem/resid/2005-2006/annue.shtml#esCasabé N, Piola L, Fuchs J, Oneto ML, Pamparato L, Basack S, Giménez R, Massaro R, PapaJC & Kesten E. 2007: Ecotoxicological assessment of the effects of glyphosate and chlorpyrifosin an Argentine soya field. Journal of Soils and Sediments, 7: 232-239.Cavalcante DGSM, Martinez CBR & Sofia SH. 2008. Genotoxic effects of Roundup on the fishProchilodus lineatus. Mutation Research 655: 41-6.Cavaş T & Könen S. 2007. Detection of cytogenetic and DNA damage in peripheral erythrocytesof goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus testand the comet assay. Mutagenesis 22: 263-8.Cerdeira AL & Duke SO. 2006. The current status and environmental impacts of glyphosate-resistant crops: a review. Journal of Environmental Quality 35:1633-58.Chang C-B & Chang C-C. 2009. Refractory cardiopulmonary failure after glyphosate surfactantintoxication: a case report. Journal of Occupational Medicine and Toxicology 4: 2.Chin-hua M & Palada MC. 2006. Fertility management of the soil-rhizosphere system forefficient fertilizer use in vegetable production. Match 2011 http://www.agnet.org/activities/sw/2006/794098668/Clements C, Ralph S & Petras M. 1997. Genotoxicity of select herbicides in Rana catesbeianatadpoles using the alkaline single-cell gel DNA electrophoresis (comet) assay. Environmentaland Molecular Mutagenensis 29: 277-88.Costa MJ, Monteiro DA, Oliveira-Neto AL, Rantin FT & Kalinin AL. 2008. Oxidative stressbiomarkers and heart function in bullfrog tadpoles exposed to Roundup Original. Ecotoxicolgy173: 153-63.Cox C. 2004. Glyphosate. Journal of Pesticide Reform 24: 10-15.De Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF & Blair A.2003. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphomaamong men. Occupational and Environmental Medicine 60: E11.De Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, Sandler DP & Alavanja MC.2005. Cancer incidence among glyphosate-exposed pesticide applicators in the AgriculturalHealth Study. Environmental Health Perspectives 113: 49-54.Delmas A-D. 2004. Environmental Stewardship programme at the watershed scale - Impacton glyphosate transfer to surface water. January 2011 See http://www.egeis.org/home/monitoring/case_studies.php?article_id=15DG SANCO. 2002. Working Document Guidance Document on Terrestrial Ecotoxicology UnderCouncil Directive 91/414/EEC. http://www.pesticides.gov.uk/psd_pdfs/registration_guides/data_reqs_handbook/Supporting/Terrestrial-Ecotox-rev2_final.pdfDucic T & Polle A. 2005. Transport and detoxication of copper and manganese in plants.Brazilian Journal of Plant Physiology 17: 103-112.Duke SO. 2005. Taking stock of herbicide-resistant crops ten years after introduction. PestManagement Science 61: 211–218Duke SO & Powles SB. 2008 Glyphosate: a once-in-a-century herbicide. Pest ManagementScience. 64: 319-325.EFSA (European Food Safety Agency). 2009. 2007 Annual Report on Pesticide Residuesaccording to Article 32 of Regulation (EC) No 396/2005. http://www.efsa.europa.eu/en/scdocs/scdoc/305r.htmEl-Shenawy NS. 2009. Oxidative stress responses of rats exposed to Roundup and its activeingredient glyphosate. Environmental Toxicology Pharmacology 28: 39-85.GM Freeze and Greenpeace| GRL-TN 03/2011 |June 201139
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Eriksson M, Hardell L, Carlberg M & Akerman M. 2008. Pesticide exposure as risk factor fornon-Hodgkin lymphoma including histopathological subgroup analysis. International Journal ofCancer 123: 1657-63.European Union Council Directive 98/83/EC on the quality of drinking water intended for humanconsumption.Gallardo L. 2001. Aerial herbicide impact on farmers in Ecuador. Pesticide News 54: 8.Garry VF, Harkins ME, Erickson LL, Long-Simpson LK, Holland SE & Burroughs BL. 2002. Birthdefects, season of conception, and sex of children born to pesticide applicators living in the RedRiver Valley of Minnesota, USA. Environmental Health Perspectives 110: 441-9.Gasnier C, Benachour N, Clair E, Travert C, Langlois F, Laurant C, Decroix-Laporte C & SéraliniGE. 2010. Dig1 protects against cell death provoked by glyphosate-based herbicides in humanliver cell lines. Journal of Occupational Medicine and Toxicology 5: 29.Gehin A, Guillaume YC, Millet J, Guyon C & Nicod L. 2005 Vitamins C and E reverse effect ofherbicide-induced toxicity on human epidermal cells HaCaT: a biochemometric approach.International Journal of Pharmaceutics 288: 219-226.Gehin A, Guyon C & Nicod L. 2006. Glyphosate-induced antioxidant imbalance in HaCaT: theprotective effect of vitamins C and E. Environmental Toxicology and Pharmacology 22: 27-34.Geisy JP, Dobson S & Solomon KP. 2000. Ecotoxicological risk assessment for roundupherbicide. Reviews of Environmental Contamination and Toxicology 167: 35-120.Gimsing AL, Borggaard OK & Bang M. 2004. Influence of soil composition on adsorption ofglyphosate and phosphate by contrasting Danish surface soils, European Journal of Soil 55:183-191.Gimsing AL, Borggaard OK, Jacobsen OS, Aamand J & Sorensen J. 2004. Chemical andmicrobiological soil characteristics controlling glyphosate mineralisation in Danish surface soils.Applied Soil Ecology 27: 233-242.Glusczak L, Dos Santos Miron D, Crestani M, Da Fonseca MB, De Araújo Pedron F, DuarteMF & Vieira VLP. 2006. Effect of glyphosate herbicide on acetylcholinesterase activity andmetabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicology andEnvironmental Safety 65: 237-241.Glusczak L, Dos Santos Miron D, Moraes BS, Simões RR, Schetinger MRC, Morsch VM & LoroVL. 2007. Acute effects of glyphosate herbicide on metabolic and enzymatic parameters ofsilver catfish (Rhamdia quelen) Comparative Biochemistry and Physiology Part C 146: 519-524.Goldstein DA, Acquavella JF, Manion RM & Farmer DR. 2002. An analysis of glyphosate datafrom the California Environmental Protection Agency Pesticide Illness Surveillance Program.Journal of Toxicology - Clinical Toxicology 40: 885-92.Gordon B. 2007. Manganese nutrition of glyphosate resistant and conventional soybeans (NorthAmerica). Better Crops with Plant Food 91: 12-13.Granby K & Vahl M. 2001. Investigation of the herbicide glyphosate and the plant growthregulators chlormequat and mepiquat in cereals produced in Denmark. Food Additives andContamination 18: 898-905.Green JM, Hazel CB, Forney DR & Pugh LM. 2008. New multiple-herbicide crop resistance andformulation to augment the utility of glyphosate. Pest Management Science 64: 332-339.Grisolia CK. 2002. A comparison between mouse and fish micronucleus test usingcyclophosphamide, mitomycin C and various pesticides. Mutation Research 518: 145-50.Grue CE, Gibert PL & Seeley ME. 1997. Neurophysiological and behavioral changes in non-target wildlife exposed to organophosphate and carbamate pesticides: thermoregulation, foodconsumption and reproduction. American Zoologist 37: 369-388.Guilherme S, Gaivao I, Santos MA & Pacheco M. 2009. Tissue specific DNA damage inthe European eel (Anguilla anguilla) following a short-term exposure to a glyphosate-basedherbicide. Toxicological Letters 189S:S212:Z15.Haney RL, Senseman SA, Hons FM & Zuberer DA. 2000. Effect of glyphosate on soil microbialactivity and biomass. Weed Science 49: 89-93.Hardell L & Eriksson M. 1999. A case-control study of non-Hodgkin lymphoma and exposure topesticides. Cancer 85: 1353-60.Hardell L, Eriksson M & Nordstrom M. 2002. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: pooled analysis of two Swedish case-controlstudies. Leukaemia Lymphoma 43: 1043-9.Heard MS, Hawes C, Champion GT, Clark SJ, Firbank LG, Haughton AJ, Parish AM, Perry JN,Rothery P, Scott RJ, Skellern MP, Squire GR & Hill MO. 2003a. Weeds in fields with contrastingconventional and genetically modifies herbicide-tolerant crop – I. Effects on abundance anddiversity. Philosophical Transactions of The Royal Society London B 358: 1819-1832.Heard MS, Hawes C, Champion GT, Clark SJ, Firbank LG, Haughton AJ, Parish AM, Perry JN,Rothery P, Scott RJ, Skellern MP, Squire GR & Hill MO. 2003b. Weeds in fields with contrastingconventional and genetically modifies herbicide-tolerant crops. II. Effects on individual species.
Philosophical Transactions of The Royal Society London B 358: 1833-1846.Hokanson R, Fudge R, Chowdhary R & Busbee D. 2007. Alteration of estrogen-regulated geneexpression in human cells induced by the agricultural and horticultural herbicide glyphosate.Human and Experimental Toxicology 26: 747-52.Huber DM, Cheng MW & Winsor BA. 2005. Association of severe Corynespora root rot ofsoybean with glyphosate-killed giant ragweed. Phytopathology 95: S45.Huber D. (Undated). AG Chemical and Crop Nutrient Interactions – Current Update. November2010 http://nfuontario.ca/upload/files/userfiles/Glyphosate%20and%20Crop%20Nutrient%20Interactions.pdfHumphries D, Brytus G & Anderson AM. 2005. Glyphosate residues In Alberta’s atmosphericdeposition, soils and surface waters. Water Research Users Group Alberta Environment.IAASTD (International Assessment of Agricultural Knowledge, Science and Technology forDevelopment). 2009a. Volume IV: North America and Europe. Chapter 3, Environmental,Economic and Social Impacts of NAE Agriculture and AKST. Island Press, Washington DC.IAASTD (International Assessment of Agricultural Knowledge, Science and Technologyfor Development). 2009b. Synthesis Report Island Press, Washington DC. http://www.agassessment.org/reports/IAASTD/EN/Agriculture%20at%20a%20Crossroads_Synthesis%20Report%20(English).pdfInternational Survey of Herbicide Resistant Weed. March 2011 http://www.weedscience.org/Summary/UspeciesMOA.asp?lstMOAID=12&FmHRACGroup=GoInternational Survey of Herbicide Resistant Weeds. March 2011. http://www.weedscience.org/Case/Case.asp?ResistID=5344Iowa University State Extension. 2003. Protecting Iowa’s rare and endangered plants. http://www.extension.iastate.edu/Publications/PM1506.pdfIPCS. 1994. Environmental Health Criteria 159: Glyphosate. International Programme onChemical Safety, World Health Organisation, Geneva. http://www.inchem.org/documents/ehc/ehc/ehc159.htm.ISAAA. 2011. ISAAA brief 42-2010. Executive Summary. Global status of commercializedbiotech/GM crops: 2010. http://www.isaaa.org/resources/publications/briefs/42/executivesummary/default.aspJohal GS & Huber DM. 2009. Glyphosate effect on plant diseases. European Journal ofAgronomy 31: 144-152.Johnson WG, Owen MDK, Kruger GR, Young BG, Shaw DR, Wilson RG, Wilcut JW, JordanDL & Weller SC. 2009. US farmer awareness of glyphosate-resistant weeds and resistancemanagement strategies. Weed Technology 23: 308–312.Kale PG, Petty BT Jr., Walker S, Ford JB, Dehkordi N, Tarasia S, Tasie BO, Kale R & SohniYR. 1995. Mutagenicity testing of nine herbicides and pesticides currently used in agriculture.Environmental and Molecular Mutagenisis 25: 148-53.Kaya B, Creus A, Yanikoğlu A, Cabré O & Marcos R. 2000. Use of the Drosophila wing spot testin the genotoxicity testing of different herbicides. Environmental and Molecular Mutagenisis 36:40-6.Kelly DW, Poulin R, Tompkins DM & Townsend CR. 2010. Synergistic effects of glyphosateformulation and parasite infection on fish malformations and survival. Journal of Applied Ecology47: 498–504.Keuger J. 2005. Pesticide occurrence in Swedish surface waters The Vinne catchment study.http://www.egeis.org/home/monitoring/case_studies.php?article_id=92Kjær J, Ullum M, Olsen P, Sjelborg P, Helweg A, Mogensen B, Plauborg F, Grant R, FomsgaardI & Brüsch W. 2003. The Danish pesticide leaching assessment programme monitoring resultsMay 1999–June 2002 Third report. Geological Survey of Denmark and Greenland.Kjær J, Olsen P, Ullum M & Grant R. 2005. Leaching of glyphosate and amino-methylphosphonicacid from Danish agricultural field sites Journal of Environmental Quality 34: 608–620Kremer RJ & Means NE. 2009. Glyphosate and glyphosate-resistant crop interactions withrhizosphere microorganisms. European Journal of Agronomy 31: 153-161.Kristoffersen P, Rask AM, Grundy AC, Franzen I, Kempenaar C, Raisio J, Schroeder H, SpijkerJ, Verschwele A & Zarina L. 2008. A review of pesticide policies and regulations for urbanamenity areas in seven European countries. Weed Research 48(3) published on-line. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-3180.2008.00619.x/pdfKuklinsky-Sobral J, Araujo WL, Mendes R, Pizzirani-Kleiner AA & Azevedo JL. 2005. Isolationand characterization of endophytic bacteria from soybean (Glycine max) grown in soil treatedwith glyphosate herbicide. Plant and Soil 273: 91-99.Lajmaonovich RC, Attademo AM, Peltzer PM, Junges CM & Cabagna MC. 2011. Toxicity offour herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles:B-esterases and glutathione S-transferase Inhibitors. Archives of Environmental Contaminationand Toxicology 60: 681-689
40GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
Lioi MB, Scarfi MR, Santoro A, Barbieri R, Zeni O, Salvemini F, Di Berardino D & Ursini MV.1998a. Cytogenetic damage and induction of pro-oxidant state in human lymphocytes exposedin vitro to gliphosate, vinclozolin, atrazine, and DPX-E9636. Environmental and MolecularMutagenisis 32:39-46.Lioi MB, Scarfì MR, Santoro A, Barbieri R, Zeni O, Di Berardino D & Ursini MV. 1998b.Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte culturesin vitro. Mutational Research 403: 13-20.Lucas S. 2006. Biotech increases pesticide use in Brazil. January 2011 http://www.farmchemicalsinternational.com/news/marketupdates/index.php?storyid=296&style=1Mallory-Smith C & Zapoila M. 2008. Gene flow from glyphosate-resistant crops. PestManagement Science 64: 428-440.Mañas F, Peralta L, Raviolo J, García Ovando H, Weyers A, Ugnia L, Gonzalez Cid M, LarripaI & Gorla N. 2009a. Genotoxicity of glyphosate assessed by the comet assay and cytogenetictests. Environmental Toxicology and Pharmacology 28: 37-41.Mañas F, Peralta L, Raviolo J, García Ovando H, Weyers A, Ugnia L, Gonzalez Cid M, Larripa I &Gorla N. 2009b. Genotoxicity of AMPA, the environmental metabolite of glyphosate, assessedby the Comet assay and cytogenetic tests. Ecotoxicology and Environmental Safety 72: 834-7.Marc J, Le Breton M, Cormier P, Morales J, Belle R & Mulner-Lorillo O. 2005. A glyphosate-based pesticide impinges on transcription. Toxicology and Applied Pharmacology 203: 1-8.Marc J, Mulner-Lorillon O & Bellé R. 2004. Glyphosate-based pesticides affect cell cycleregulation. Biology of the Cell 96: 245-9.Marc J, Mulner-Lorillon O, Durand G & Bellé R. 2003. Embryonic cell cycle for risk assessmentof pesticides at the molecular level. Environmental Chemistry Letters 1: 8-12.Marc J, Mulner-Lorillon O, Boulben S, Hureau D, Durand G & Bellé R. 2002. Pesticide Roundupprovokes cell division dysfunction at the level of CDK1/cyclin B activation. Chemical Research inToxicology 15: 326-31.Matarczyk JA, Willis JA, Vranjic JA & Ash JE. 2002. Herbicides, weeds and endangeredspecies: management of bitou bush (Chrysanthemoides monilifera ssp. rotundata) withglyphosate and impacts on the endangered shrub, Pimelea spicata. Biological Conservation108: 133-141.McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D,Skinnider LF & Choi NW. 2001. Non-Hodgkin’s lymphoma and specific pesticide exposuresin men: cross-Canada study of pesticides and health. Cancer, Epidemiology, Biomarkers andPrevention 10: 1155-63.Monsanto. 1982. Materials Safety Data. http://www.stcloudstate.edu/osh/msds/documents/GlyphosateTechnical.pdfMonsanto. 2003. Backgrounder. Glyphosate and water quality. Updated November 2003.http://www.monsanto.com/products/Documents/glyphosate-background-materials/gly_water_bkg.pdfMonsanto. 2005a. Backgrounder. History of Monsanto’s glyphosate herbicide. http://www.monsanto.com/products/Documents/glyphosate-background-materials/back_history.pdfMonsanto. 2005b. Backgrounder. Glyphosate half life in the soil. Backgrounder November2010. http://www.monsanto.com/products/Documents/glyphosate-background-materials/gly_halflife_bkg.pdfMonsanto. 2008. Management guide for maretails. March 2010 http://www.monsanto.com/weedmanagement/Documents/Marestail.pdfMonsanto 2009. Corn, R&D Pipeline/ Discovering. Delivering. Yielding. February 2011http://www.monsanto.com.ar/biotecnologia/documentos/pipeline_2009.pdfMonsanto 2010a. Backgrounder. Response to the study: The impacts of insecticides andherbicides on the productivity and biodiversity of aquatic communities. http://www.monsanto.com/products/Documents/glyphosate-background-materials/bkg_amphib_05a.pdfMonsanto. 2010b. Roundup. http://www.monsanto.com.au/products/roundup/default.aspMonsanto. 2010c. BASF and Monsanto announce progress in Dicamba formulations. 2 Nov,2010 Press release. http://monsanto.mediaroom.com/index.php?s=43&item=897Monsanto. 2011. Technology Use Guide 2011. http://www.monsanto.com/SiteCollectionDocuments/2011_TUG_021011.pdf. See also March 2011 http://www.monsanto.com/Documents/2010_technology_use_guide.pdfMonsanto. (Undated). Glyphosate resistant weed biotypes. http://www.monsanto.com/weedmanagement/Pages/Glyphosate-ResistantWeedBiotypes.aspx. Accessed March 2011.Mose T, Kjaerstad MB, Mathiesen L, Nielsen JB, Edelfors S & Knudsen LE. 2008. Placentalpassage of benzoic acid, caffeine, and glyphosate in an ex vivo human perfusion system.Journal of Toxicology and Environmental Health A 71: 984-91.Mueckay C & Maldonado A. 2003. Daños genéticos en la frontera de Ecuador por las
fumigaciones del Plan Colombia. Acción Ecológica, Acción Creativa, Clinica de DerchosHumanos de la PUCE, CAS, CEDHU, CONAIE, FORCCOFES, INREDH, Plan Pais, RAPALEcuador, SERPAJ Ecuador. http://www.visionesalternativas.com/militarizacion/articulos/pcolom/AE0311.pdf.National Agricultural Statistics Service. 2009. Acreage. February 2011 http://usda.mannlib.cornell.edu/usda/nass/Acre/2000s/2009/Acre-06-30-2009.pdfNelson PE, Toussoun TA & Marasas WFO. 1983. Fusarium species. An illustrated manual foridentification. Pennsylvania State University Press, USA.Nordstrom M, Hardell L, Magnuson A, Hagberg H & Rask-Anderson A. 1998. Occupationalexposures, animal exposure and smoking as risk factors for hairy cell leukaemia evaluated in acase-control study. British Journal of Cancer 77: 2048-52.Oldham, J. & Massey, R. 2002. Health and environmental effects of herbicide spray campaignsin Colombia. Institute for Science and Interdisciplinary Studies, Amherst MA, USA. http://www.laslianas.org/Colombia/SprayingReview_Oldham-Massey.pdfOtaño A, Correa B & Palomares S. 2010. Water Pollutants Investigation Committee – FirstReport. http://www.gmwatch.eu/files/Chaco_Government_Report_Spanish.pdf.Paganelli A, Gnazzo V, Acosta H, López SL & Carrasco AE. 2010. Glyphosate-based herbicidesproduce teratogenic effects on vertebrates by impairing retinoic acid signalling. ChemicalResearch in Toxicology 23: 1586-95.Papadopoulou-Mourkidou E. 2004. Glyphosate monitoring in Greek surface waters. http://www.egeis.org/home/monitoring/case_studies.php?article_id=7Paz-y-Miño C, Sánchez ME, Arévalo M, Muñoz MJ, Witte T, De-la-Carrera GO & Leone PE.2007. Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate. Geneticsand Molecular Biology 30: 456-60.Peluso M, Munnia A, Bolognesi C & Parodi S. 1998. 32P-postlabeling detection of DNAadducts in mice treated with the herbicide Roundup. Environmental and Molecular Mutagenesis31: 55-9.Pérez GL, Torremorell A, Mugni H, Rodríguez P, Solange Vera M, Do Nascimento M, AllendeL, Bustingorry J, Escaray R, Ferraro M, Izaguirre I, Pizarro H, Bonetto C, Morris DP & ZagareseH. 2007. Effects of the herbicide Roundup on freshwater microbial communities: a mesocosmstudy. Ecological Applications 17: 2310-22.Perry JN, Firbank LG, Champion GT, Clark SJ, Heard MS, May MJ, Hawes C, Squire GR,Rothery P, Woiwod IP & Pidgeon JD. 2004. Ban on triazine herbicides likely to reduce but notnegate relative benefits of GMHT maize cropping. Nature 428: 313-316.Pesticide Residues Committee. 2007a. Pesticide Residues Monitoring Report. Fourth Quarterreport 2006.Pesticide Residues Committee. 2007b. Minutes of the Meeting of the Pesticide ResiduesCommittee 10th May 2007. http://www.pesticides.gov.uk/prc.asp?id=2206Pesticide Residues Committee. 2008. Pesticide Residues Monitoring Report. Third QuarterReport.Pesticide Residues Committee. 2009. Pesticide Residues Monitoring Report. Fourth QuarterReport.Pesticide Residues Committee. 2010. Pesticide Residues Monitoring Report. Fourth QuarterReport.Pieniążek D, Bukowska B & Duda W. 2004. Comparison of the effect of Roundup Ultra 360 SLpesticide and its active compound glyphosate on human erythrocytes. Pesticides, Biochemistryand Physiology 79: 58-63.Poletta GL, Larriera A, Kleinsorge E & Mudry MD. 2009. Genotoxicity of the herbicideformulation Roundup� (glyphosate) in broad-snouted caiman (Caiman latirostris) evidenced bythe Comet assay and the Micronucleus test. Mutatation Research 672: 95-102.Potrebic O, Jovic-Stosic J, Vucinic S, Tadic J & Radulac M. 2009. [Acute glyphosate-surfactantpoisoning with neurological sequels and fatal outcome]. Vojnosanit Pregl 66:758-62.Powles SB. 1998. Evolved resistance to glyphosate in rigid ryegress (Loliumregida) in Australia.Weed Science 46: 604-607.Powles SB. 2008. Evolved glyphosate-resistant weeds around the world: lessons learnt. PestManagement Science 64: 360-365.Powell JR & Swanton CJ. 2008. A critique of studies evaluating glyphosate effects on diseasesassociated withFusariumspp. Weed Research 48, 307–318Rank J, Jensen A-G, Skov B, Pedersen LH & Jensen K. 1993. Genotoxicity testing of theherbicide Roundup and its active ingredient glyphosate isopropylamine using the mouse bonemarrow micronucleus test, Salmonella mutagenicity test, and Allium anaphase-telophase test.Mutatation Research 300: 29-36.Relyea RA. 2005a. The lethal impacts of Roundup and predatory stress on six species of NorthGM Freeze and Greenpeace| GRL-TN 03/2011 |June 201141
Herbicide tolerance and GM cropsWhy the world should beReady to Round Up glyphosate
American tadpoles. Archives of Environmental Contamination and Toxicology 48: 351-357.Relyea RA. 2005b. The impact of insecticides and herbicides on the biodiversity andproductivity of aquatic communities. Ecological Applications, 15: 618–627.Relyea RA. 2005c. The lethal impact of roundup on aquatic and terrestrial amphibians.Ecological Applications 15: 1118–1124.Richard S, Moslemi S, Sipahutar H, Benachour N & Séralini GE. 2005. Differential effects ofglyphosate and Roundup on human placental cells and aromatase. Environmental HealthPerspectives 113: 716-20.Rom WN & Markowitz SB. 2007. Environmental and Occupational Medicine 4th Edition,Chapter 74 p 1170. Lippincott,Williams and Wilkins, Philadelphia, USA.Romano RM, Romano MA, Bernardi MM, Furtado PV & Oliveira CA. 2010. Prepubertalexposure to commercial formulation of the herbicide glyphosate alters testosterone levels andtesticular morphology. Archives of Toxicology 84: 309–317.Rosenbom AE, Brüsch W, Juhler RK, Ernstsen V, Gudmundsson L, Kjær J, Plauborg F, GrantR, Nyegaard P & Olsen P. 2010. The Danish Pesticide Leaching Assessment ProgrammeMonitoring results May 1999–June 2009. Geological Survey of Denmark and Greenland,Ministry of Climate and Energy and Faculty of Agricultural Sciences.Roy DB, Bohan DA, Haughton AJ, Hill MO, Osborne JL, Clark SJ, Perry JN, Rothery P, ScottRJ, Brooks DR, Champion GT, Hawes C, Heard MS & Firbank LG. 2003. Invertebrates andvegetation of the field margins adjacent to crops subject to contrasting herbicides regimes inthe Farm Scale Evaluations of genetically modified herbicide –tolerant crops. PhilosophicalTranslations of The Royal Society London B 358: 1879-1898.Saltimiras D, Bus JS, Spanogle T, Hauswirth J, Tobia A & Hill S. 2011. Letter to the EditorRegarding the Article by Paganelli et al. Chemical Research in Toxicology. DOI: 10.1021/tx100452kSavitz DA, Arbuckle T, Kaczor D & Curtis KM. 1997. Male pesticide exposure and pregnancyoutcome. American Journal of Epidemiology 146: 1025-36.Schullz A, Krüper A & Amrhein N. 1985. Differential sensitivity of bacterial5-enolpyruvylshikimate-3-phosphate synthases to the herbicide glyphosate. FEMS MicrobialLetters 28: 297-301.Scott, L. 2006. Biotech increases glyphosate use in Brazil. January 2011 http://www.farmchemicalsinternational.com/news/marketupdates/?storyid=296Serra AP, Marchetti ME, Candido ACS, Dia ACR & Christoffoleti PJ. 2011. Influência do glifosatona eficiência nutricional do nitrogênio, manganês, ferro, cobre e zinco em soja resistente aoglifosato. Ciência Rural 41: 77-84.Service RF. 2007. A growing threat down on the farm. Science 316: 1114-1117.Shushkova T, Ermakova I & Leontievsky A. 2009. Glyphosate bioavailability in the soil.Biodegradation 21: 403-410.Simonsen L, Fomsgard IS, Svensmark B & Splid NH. 2008. Fate and availability of glyphosateand AMPA in agricultural soil. Journal of Environmental Science and Health Part B 43: 365-375.Siviková K. & Dianovský J. 2006. Cytogenetic effect of technical glyphosate on cultivated bovineperipheral lymphocytes. International Journal of Hygiene and Environmental Health 209: 15-20.Slager RE, Poole JA, Levan TD, Sandler DP, Alavanja MC & Hoppin JA. 2009. Rhinitisassociated with pesticide exposure among commercial pesticide applicators in the AgriculturalHealth Study. Occupational and Environmental Medicine 66: 718-24.Springett AJ & Gray RAJ. 1992. Effect of repeated low doses of biocides on the earthwormAporrectodea caliginosa in laboratory culture. Soil Biology and Biochemistry 24: 1739-1744.Stachowski-Haberkorn S, Becker B, Marie D, Haberkorn H, Coroller L & De la Broise D. 2008.Impact of Roundup on the marine microbial community, as shown by an in situ microcosmexperiment. Aquatic Toxicity 89: 232-241.Stride C. 2010. The agronomic benefits of glyphosate in Europe. Review of the benefits ofglyphosate per market use. MonsantoSzarek J, Siwicki A, Andrzejewska A, Terech-Majewska E & Banaszkiewicz T. 2000. Effects ofthe herbicide Roundup on the ultrastructural pattern of hepatocytes in carp (Cyprinus carpio).Marine Environmental Research 50: 263-266.Tesfamariam T, Bott S, Cakmak I, Römheld V & Neumann G. 2009. Glyphosate in therhizosphere – role of waiting times and different glyphosate binding forms in soils andphytotoxicity to non-target plants. European Journal of Agronomy 31: 126-131.The Crop Site. 2010. Brazil launches non-GM soy research plan. http://www.thecropsite.com/news/7153/brazil-launches-nongm-soy-research-planTillman D. 1999. Global environmental impacts of agricultural expansion: The need for sustainableand efficient practices. Proceeding of the National Academy of Sciences of the United Sates ofAmerica 96:5995-6000.
US EPA (Environmental Protection Agency). 2009. Edition of the Drinking Water Standards forhealth and Advisories EPA 822-R-09-011 Office of Water US Environmental Protection AgencyWashington, DCUS EPA (Environmental Protection Agency). 1993. EPA Reregistration Eligibility Document,Glyphosate. EPA 738-R-93-014. Office of Prevention, Pesticides and Toxic Substances, UnitedStates Environmental Protection Agency. Washington DC.US EPA (Environmental Protection Agency). 2006. Memorandum: Glyphosate Human HealthRisk Assessment for Proposed Use on Indian Mulberry and Amended Use on Pea, Dry. PCCode: 417300, Petition No: 5E6987, DP Num: 321992, Decision No. 360557. From TomerlinJR, Alternative Risk Integration and Assessment Team (ARIA) Fungicide Branch, RegistrationDivision. Office of Prevention, Pesticides and Toxic Substances, United States EnvironmentalProtection Agency. Washington, D.C. http://www.regulations.gov. under Docket No. EPA-HQ-OPP-2006-0177.USFDA. 2008. Pesticide Program Pesticide Monitoring Database 2008. March 2011http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Pesticides/ResidueMonitoringReports/ucm229204.htmValverde B & Gressel J. 2006. Dealing with evolution and spread of Sorghum halepenseglyphosate resistances and spread in Argentina. A consultancy report to SENASA . March2011. www.weedscience.org/paper/Johnsongrass%20Glyphosate%20Report.pdfVan Gessel MJ. 2001. Glyphosate-resistant horseweed from Delaware. Weed Science 49:703-705.Vera MS, Lagomarsino L, Sylvester M, Pérez GL, Rodríguez P, Mugni H, Sinistro R, Ferraro M,Bonetto C, Zagarese H & Pizarro H. 2010. New evidences of Roundup (glyphosate formulation)impact on the periphyton community and the water quality of freshwater ecosystems.Ecotoxicology 19: 710-721.Vigfusson NV & Vyse ER. 1980. The effect of the pesticides Dexon, Captan, and Roundup, onsister-chromatid exchanges in human lymphocytes in vitro. Mutation Research 79: 53-7.Vila Aiud MM, Vidal RA, Balbi MC, Gundel PE, Trucco F & Ghersa CM. 2008. Glyphosate-resistant weeds of South American cropping systems: an overview. Pest Management Science64: 366-371.Walsh LP, McCormick C, Martin C & Stocco DM. 2000. Roundup inhibits steroidogenesis bydisrupting steroidogenic acute regulatory (StAR) protein expression. Environmental HealthPerspectives 108: 769-76.Wardle DA & Parkinson D. 1990. Effects of three herbicides on soil microbial biomass andactivity. Plant Science 122: 21-28.WHO. 2005. Glyphosate and AMPA in Drinking-water. Background document for developmentof WHO Guidelines for Drinking-water QualityWHO/FAO. 2005. Pesticide residues in food .FAO Plant Production and Protection paper103.Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Foodand the Environment and the WHO Core Assessment Group on Pesticide Residues Geneva,Switzerland, 20–29 September 2005. http://www.fao.org/ag/AGP/AGPP/Pesticid/JMPR/DOWNLOAD/2005_rep/report2005jmpr.pdfWilliams BK & Semlitsch RD. 2010. Larval Responses of Three Midwestern Anurans to Chronic,Low-Dose Exposures of Four Herbicides. Archives of Environmental Contamination andToxicology 58: 819-827.Williams N. 2004. Fears grow for amphibians. Current Biology 14: 986-987.Yamada T & Castro PR. 2005. Glifosato, herbicida com singular modo de ac� ão:Efeitossecundários e implicac� ões fisiológicas e agronômicas. http://www.ipni.net/ppiweb/pbrazil.nsf/b2aaf15da221a95785256a6d006d7a23/425d07bd384d51950325704a004dbe75/$FILE/Anais%20Yamada%20e%20Paulo%20Castro.pdfYasmin S & D’Souza D. 2007. Effect of pesticides on the reproductive output of Eisenia fetida.Bulletin of Environmental Toxicology and Contamination 79: 529-532.Zablowicz RM & Reddy KN. 2004. Impact of glyphosate on the Bradyrhizobium japonicumsymbiosis with glyphosate-resistant transgenic soybean: a minireview. Journal of EnvironmentalQuality 33: 825-831Zelaya IA, Owen MDK & Van Gessel MJ. 2004. Inheritance of evolved glyphosate resistance inConyza canadensis (L.) Cronq. Theoretical and Applied Genetics 110: 58–70.Zobiole LHS, Kremer RJ, Oliveira RS, Constantin J. 2011a. Glyphosate affects chlorophyll,nodulation and nutrient accumulation of “second generation” glyphosate-resistant soybean(Glycine max L.) Pesticide Biochemistry and Physiology 99: 53-60.Zobiole LHS, Kremer RJ, Oliveira RS, Constantin J. 2011b. Glyphosate affects micro-organisms inrhizospheres of glyphosate-resistant soybeans Journal of Applied Microbiology. 110: 118-127.
42GM Freeze and Greenpeace| GRL-TN 03/2011 |June 2011
� GREENPEACE / ALEXANDRA BUXBAUM
No GM herbicide-tolerant cropcan form partof a sustainableagriculture model,and the cultivationof all such cropsshould be bannedGM Freeze and Greenpeace| GRL-TN 03/2011 |June 201143
Published byGreenpeace InternationalOttho Heldringstraat 51066 AZ AmsterdamThe Netherlandsgreenpeace.orgGreenpeace Research LaboratoriesInnovation Centre 2Rennes DriveUniversity of ExeterUK EX4 4RNGM FreezeRegistered Office50 South Yorkshire BuildingsSilkstone CommonBarnsleyUK S75 4RJgmfreeze.orgFor more information contact:enquiries@greenpeace.orgenquiry@gmfreeze.org
greenpeace.orggmfreeze.org