Miljø- og Planlægningsudvalget 2010-11 (1. samling)
MPU Alm.del Bilag 733
Offentligt
Survey on basic knowledge aboutexposure and potentialenvironmental and health risks forselected nanomaterialsSonja Hagen Mikkelsen, Erik Hansen andTrine Boe ChristensenCOWI A/S, DenmarkAnders Baun and Steffen Foss HansenDTU EnvironmentMona-Lise BinderupDTU Food
Environmental ProjectNo. 13702011Miljøprojekt
The Danish Environmental Protection Agency will, when opportunityoffers, publish reports and contributions relating to environmentalresearch and development projects financed via the Danish EPA.Please note that publication does not signify that the contents of thereports necessarily reflect the views of the Danish EPA.The reports are, however, published because the Danish EPA finds thatthe studies represent a valuable contribution to the debate onenvironmental policy in Denmark.
Table of ContentsPREFACEEXECUTIVE SUMMARYINTRODUCTION1.11.21.3OVERVIEW OF TYPES OF NANOMATERIALSNANOMATERIALS IN CONSUMER PRODUCTSSPECIAL CHARACTERISTICS OF NANOMATERIALS VS.BULKMATERIALSUSE OF NANOMATERIALS INDENMARK
7913131516181819222323242425252627272727272828293032333333343536363637383839404343
1.41.4.1Industry and products1.4.2Results from selected Nordic surveys1.5 INDUSTRY SURVEY2NANOMATERIALS SURVEY
2.1 SELECTION OF NANOMATERIALS FOR THE SURVEY2.2 NANOMATERIALS PROFILES2.2.1Manufacturing and applications2.2.2Ecotoxicological and toxicological profiles2.2.3Relevant exposures2.2.4Risk profiles3FULLERENES - C603.1 GENERAL CHARACTERISTICS3.2 MANUFACTURING PROCESSES3.3 USES3.3.1Main applications3.3.2Results from industry survey3.4 ECO-TOXICOLOGICAL PROFILE3.5 TOXICOLOGICAL PROFILE3.5.1ADME studies3.5.2Short term toxicity3.5.3Irritation and corrosion3.5.4Skin and respiratory sensitisation3.5.5Repeated dose toxicity3.5.6Mutagenotoxicity/genotoxicity3.5.7Carcinogenicity3.5.8Reproductive toxicity3.5.9In vitro toxicity studies3.5.10 Summary3.6 EXPOSURE SCENARIOS3.7 RISK PROFILE3.7.1Environment3.7.2Human health3.8 SUMMARY SHEET FORC604TITANIUM DIOXIDE - TIO24.1GENERAL CHARACTERISTICS
3
4.2 MANUFACTURING PROCESSES4.3 USES4.3.1Main applications4.3.2Results from industry survey4.4 ECO-TOXICOLOGICAL PROFILE4.5 TOXICOLOGICAL PROFILE4.5.1ADME studies4.5.2Short term toxicity4.5.3Irritation and corrosion4.5.4Skin and respiratory sensitization (in vitro and in vivo)4.5.5Repeated dose toxicity, short term, sub-chronic and long term4.5.6Mutagenicity/genotoxicity4.5.7Carcinogenicity4.5.8Reproductive toxicity, developmental toxicity and teratogenicity4.5.9Summary4.6 EXPOSURE SCENARIOS4.7 RISK PROFILE4.7.1Environment4.7.2Human health4.8 SUMMARY SHEET FORTIO25ZERO VALENT IRON - NZVI5.1 GENERAL CHARACTERISTICS5.2 MANUFACTURING PROCESSES5.3 USES5.3.1Main applications5.3.2Results from industry survey5.4 ECO-TOXICOLOGICAL PROFILE5.5 TOXICOLOGICAL PROFILE5.6 EXPOSURE SCENARIOS5.7 RISK PROFILE5.7.1Environment5.7.2Human health5.8 SUMMARY SHEET FOR NANO-SCALE ZERO-VALENT IRON6CERIUM DIOXIDE - CEO26.1 GENERAL CHARACTERISTICS6.2 MANUFACTURING PROCESSES6.3 USES6.3.1Main applications6.3.2Results from industry survey6.4 ECO-TOXICOLOGICAL PROFILE6.5 TOXICOLOGICAL PROFILE6.5.1Uptake of CeO2 into cells6.5.2In vitro toxicity- lung models6.5.3Dermal models6.5.4Mechanistic studies - Oxidative stress6.5.5Summary6.6 EXPOSURE SCENARIOS6.7 RISK PROFILE6.8 SUMMARY SHEET FORCEO27SILVER - AG7.17.27.3GENERAL CHARACTERISTICSMANUFACTURING PROCESSESUSES
4343434445464747484949505151525354545455595959595959596061616162626565656565656666666767686969697073737373
4
7.3.1Main applications7.3.2Results from industry survey7.4 ECO-TOXICOLOGICAL PROFILE7.5 TOXICOLOGICAL PROFILE7.5.1ADME studies7.5.2Acute toxicity7.5.3Irritation and corrosion7.5.4Sensitisation7.5.5Repeated dose toxicity7.5.6Mutagenicity7.5.7Carcinogenicity7.5.8Reproductive toxicity and developmental toxicity7.5.9Biological mechanism7.5.10 Summary7.6 EXPOSURE SCENARIOS7.7 RISK PROFILE7.7.1Environment7.7.2Human health7.8 SUMMARY SHEET FOR NANO-SILVER8NANOCLAY8.1 GENERAL CHARACTERISTICS8.2 MANUFACTURING PROCESSES8.3 USES8.3.1Main applications8.3.2Results from industry survey8.4 ECO-TOXICOLOGICAL PROFILE8.5 TOXICOLOGICAL PROFILE8.5.1In vivo studies8.5.2In vitro studies8.5.3Summary8.6 EXPOSURE SCENARIOS8.7 RISK PROFILE8.7.1Environment8.7.2Human health8.8 SUMMARY SHEET FOR NANOCLAY9SILICIUM DIOXIDE SIO29.1 GENERAL CHARACTERISTICS9.2 MANUFACTURING PROCESSES9.3 USES9.3.1Main applications9.3.2Results from industry survey9.4 ECO-TOXICOLOGICAL PROFILE9.5 TOXICOLOGICAL PROFILE9.5.1ADME studies9.5.2Short term toxicity9.5.3Irritation and corrosion9.5.4Skin and respiratory sensitization (in vitro and in vivo)9.5.5Repeated dose toxicity, short term, sub-chronic and long term9.5.6Mutagenicity/genotoxicity9.5.7Carcinogenicity9.5.8Reproductive toxicity, developmental toxicity and teratogenicity9.5.9In vitro studies9.5.10 Summary9.6 EXPOSURE SCENARIOS
737474757678787879808080818182838383878989899090909191919293939393939495959595959696979898100101101102103103103104104
5
9.7 RISK PROFILE9.7.1Environment9.7.2Human health9.8 SUMMARY SHEET FORSIO210 EXPOSURE AND RISK POTENTIAL11 REFERENCESANNEX 1ANNEX 2NANO TERMINOLOGY AND ACRONYMSCOMPANIES THAT COMMERCIALISENANOTECHNOLOGY AND / OR NANOMATERIALS IN DENMARK
105105105106109113131
135
6
PrefaceDevelopment of nanomaterials opens opportunities for new product typeswith many special technological features. There is however also expressedconcern for nanomaterials health and environmental aspects, where lack ofconcrete knowledge can be a major problem in the regulation of nanomateri-als.The Danish EPA (DEPA) has already initiated several projects which havehighlighted the nanomaterials that can be found in products on the Danishmarket (Consumer Survey No. 81, 2007 / Forbrugerprojekt nr. 81, 2007(Danish version)) and the nanomaterials used in the Danish industry (Envi-ronmental Project No. 1206, 2007).For a number of nanomaterials and products specific knowledge and experi-ence are lacking and though nanomaterials are covered by the existing chemi-cal legislation there is an ongoing debate on how risk assessments of nanoma-terials best can be carried out. Chemical control is predominantly covered bycommon EU legislation, and work is currently carried out in both the EU andthe OECD to assess whether the methods used for hazard and risk assessmentare able to handle nanomaterials or if nano-materials in certain cases possessspecific properties, such that the methods and technical tools of regulationshould be adjusted accordingly.In principle REACH also covers nanomaterials, as the regulation coverschemical substances, but work is carried out in relation to REACH in order toclarify various issues concerning the definition, identification, registration andassessment of nanomaterials.Denmark has taken several initiatives related to research and knowledge gen-eration concerning the possible environmental and health effects of nanomate-rials and there are also a number of knowledge institutions in Denmark, work-ing to examine these effects. Both Danish and foreign knowledge institutionshave contributed to build up considerable knowledge about potential expo-sures to nanomaterials and the associated risks.For the individual citizen or company a major source of current knowledge onnanomaterials is the DEPA website, which provides an overview of bothnanomaterials, nanomaterials in consumer products, current research relatedto environmental and health effects, and regulation of the materials. Turningto the individual applications of nanomaterials, knowledge on exposure andpossible health and environmental effects is to a large extent missing.DEPA has therefore initiated this project to provide an overview of the exist-ing knowledge about seven of the most common nanomaterials, their envi-ronmental and health properties, the use of those nanomaterials and the pos-sibility of exposure of humans and the environment.The Danish Environmental Protection Agency has contracted with COWIA/S in collaboration with DTU Environment and DTU Food to carry out thissurvey on basic knowledge about exposure and potential environmental andhealth risks for selected nanomaterials.
7
The study has been guided by a steering group consisting of Flemming Inger-slev, Poul Bo Larsen, Magnus Løfstedt and Katrine Bom, the Danish Envi-ronmental Protection Agency, Sonja Hagen Mikkelsen, COWI A/S, AndersBaun, DTU Environment and Mona-Lise Binderup, DTU Food.This report was prepared by, Erik Hansen and Sonja Hagen Mikkelsen (Pro-ject Manager), COWI A/S, Denmark and Anders Baun and Steffen FossHansen, DTU Environment and Mona-Lise Binderup, DTU Food. TrineBoe Christensen, COWI has contributed to the development of input for in-formation material to be presented on the Danish EPA homepage. The studywas conducted during a period from September 2010 to March 2011.
8
Executive SummaryBackground And ObjectiveDanish Environmental Protection Agency (DEPA) has initiated the study"Survey on basic knowledge about exposure and potential environmental andhealth risks for selected nanomaterials". The objective of the study is to pro-vide an overview of the applications of the most commonly used or wide-spread nanomaterials and to identify areas most likely to have health or envi-ronmental problems associated with their use.Characterisation and Selection of nanomaterialsNanomaterials are often defined as materials having one or more external di-mensions in the nanoscale (1 nm to 100 nm) or materials which are nanos-tructured (possessing a structure comprising contiguous elements with one ormore dimensions in the nanoscale but excluding any primary atomic or mo-lecular structure). There is yet no scientific consensus on the more precisecategorization of nanomaterials.Seven nanomaterials have been seleted for the study. Focus is on the core par-ticles without surface functionalisation. The seven nanomaterials are:Titanium dioxideCerium dioxideFullerenes (Carbon balls)SilverZero-valent ironSilicium dioxideNanoclaySelection was made based on expected application volumes, potential humanand environmental exposure from consumer products and the expected bio-logical effects. Carbon nanotubes are covered by another similar study, andare therefore not selected here, although they would qualify based on the se-lection criteria.Use of nanomaterials in DenmarkThere is no single source of information that provides an overview of the useof nanomaterials and products in Denmark or in the EU for that matter.Pieces of information are however available from databases and previous stud-ies initiated by DEPA. This information has in this project been reviewed to-gether with results from other studies carried out in the Nordic countries andincluding estimates on relevant consumer applications and uses of the selectednanomaterials. A considerable part of the nanomaterial-containing products
9
are found to be sold from web shops in Denmark and abroad but an increas-ing part is sold from ordinary shops.A limited industry survey on the industrial use of the selected nanomaterials inDenmark has been conducted. The objective of this survey was to confirm theuse of the nanomaterials in question in Denmark, and to develope a roughestimate of the consumption.The survey was carried out among identified actors dominating the marketsfor the selected nanomaterials and their typical applications. The relevant ac-tors were asked about the uses and the amounts of the nanomaterials. Focusfor the survey was on obtaining information for the most dominant field ofapplication and not to cover all different use areas.The outcome of our survey can be summarised as follows:Titanum dioxide, nanoclay and silicium dioxide are all materials usedin most significant quantities in Denmark.The use of nanosilver has not been confirmed, but indications existthat some products/brands may contain nanosilver.The use of cerium dioxide has not been confirmed either. It is notused by leading marked actors in Denmark.
No information was available on fullerenes and zero-valent iron.Nanomaterial profilesA profile for each of the selected materials was then developed. For each ma-terial the focus has been on the general characteristics and manufacture of thenanomaterials, their current uses (mainly focussed at consumer products),and hazard profiles (ecotoxicity and human toxicity). Furthermore the pro-files include sections discussing relevant exposures from consumer productsand considerations regarding the related risk.Each nanomaterial profile is summarised in a 'summary sheet' containing thekey findings and also emphasising areas where information is lacking. Thegeneral picture is that the specific knowledge base is limited and that moreinformation is needed for sufficient characterisation of the nanomaterials andfor illustration of the relevant (eco)toxicological endpoints. In addition moreinformation is required with regard to fate, behaviour and kinetics of the dif-ferent nanoparticles and crucial to the assessment of the relevant risks is anagreed methodology for risk assessment.Conclusive risk assessments were therefore not possible to develop within theframework of the present project. Based on the reviewed literature the sevenselected nanomaterials were not found to exhibit new and completely un-known risks to the consumer or to the environment in the current application.Products in the form of liquids or free particles are expected to give rise to thehighest exposures in the environment and to humans, particularly those liq-uids that are intended to come in direct contact with the body, and the poten-tial risk is likely to increase with increased exposure. However, as the applica-bility of the existing exposure and risk assessment methodology has been chal-
10
lenged in relation to nanomaterials, there are still areas that need to be ex-plored - especially for engineered nanomaterials.A key question in relation to risk and safety assessment of nanomaterials asraised in Stoneet al(2010) is to which extent the existing knowledge baseabout toxicity and risk related to the bulk counterparts can be used in theevaluation of the nanomaterials. In other words, it is the question of whetherthe the risk information can be scaled from bulk substances to the nano-formtaking the size of the nanoparticles into account or whether it is the small sizethat triggers the nano-specific behaviour and effects.Based on the reviewed literature there are some indications that scaling of tox-icity could be relevant for the more chemically intert materials as TiO2 andSiO2 whereas e.g. carbon-based materials like fullerenes where surface-modifications are introduced are more likely to aquire nano-specific proper-ties. This is an area that needs further clarification before firm conclusions canbe made. Relevant for this discussion is also the fact that many nanoscale par-ticles (e.g. silver, nanoclay, TiO2 and SiO2,) are naturally occurring paticlesthat have been used for decades. However, these materials may also be modi-fied with different surface coating, which can alter there physical-chemicalproperties and toxicity.
11
12
Introduction1.1Overview of types of nanomaterials
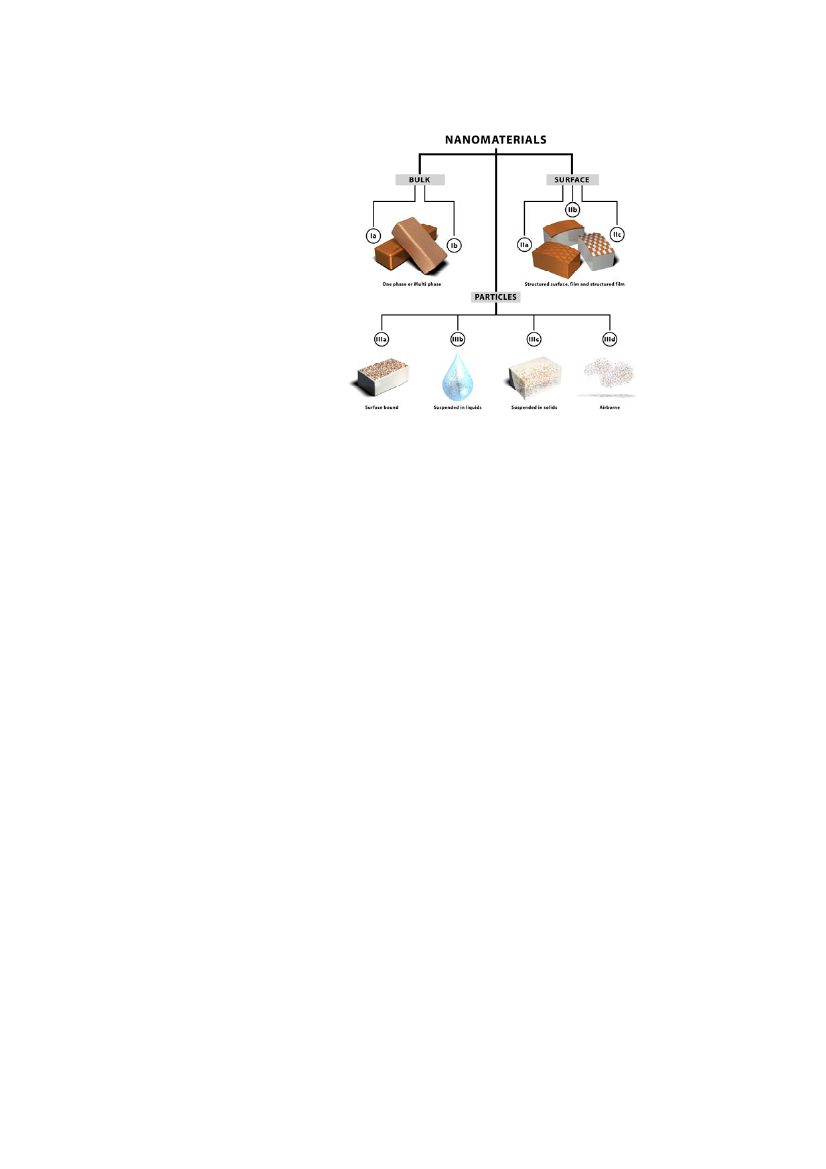
Nanoparticles can originate from primary sources (natural), from secondarysources (artificial) or they can be engineered nanoparticles.Naturally occurring nanoparticles comprise small particles from e.g. volcanicashes, particles formed during combustion processes and alsosome biologicalmolecules like RNA and DNA. Artificial nanoparticles comprise particlesfrom e.g. diesel exhaust or byproducts from industrial production whereasengineered or manufactured particles are designed with a specific purpose.Nanomaterials are often defined as materials having one or more external di-mensions in the nanoscale (1 nm to 100 nm) or materials which are nanos-tructured (possessing a structure comprising contiguous elements with one ormore dimensions in the nanoscale but excluding any primary atomic or mo-lecular structure).There is yet no scientific consensus on the more precise categorization ofnanomaterials. Hansenet al.(2007) have suggested a categorisation based onthe location of the nanoscale structure in the system, leading to a division ofnanomaterials into three main categories:I.II.III.Materials that are nanostructured in the bulk;Materials that have nanostructure on the surface; andMaterials that contain nanostructured particles.
The three main categories can be further divided into subcategories as illus-trated in Figure 1.In category I, the materials are nanostructured in three dimensions. This cate-gory is further subdivided into materials consisting of one or of two or moredifferent materials.In category II the nanostructure is on the surface and the subcategories in-clude materials where the surface is structured but surface and bulk consist ofthe same material, another subgroup is un-patterened nanoscale film on a dif-ferent substrate and the third subgroup is patterned film on a substrate wherethe film is in nanoscale thickness or the pattern is in nanoscale dimensionsalong the surface.Category III contains nanoparticles defined as free structures that arenanosized in two dimentions. The division into subcategoties depend on theenvironment around the nanoparticles, i.e. nanoparticles bound to the surfaceof a solid structure, nanoparticles suspended in a liquid, nanoparticles sus-pended in a solid, and airborne nanoparticles.
13
Figure 1 The categorization framework for nanomaterials as suggested by Hansenetal.(2007)
This categorisation closely corresponds to the classification of nanomaterialsaccording to dimensions which are not confined to the nanoscale range, wherenanomaterials can be classified as zero-dimensional (0-D), one-dimensional(1-D), two-dimensional (2-D), and three-dimensional (3-D).0-D nanomaterials are those materials where all dimensions are nanoscale,most commonly nanoparticles; 1-D nanomaterials have one dimention outsidethe nanoscale, e.g. nanotubes, -rods and -wires; 2-D nanomaterials have twodimensions outside the nanoscale, e.g. nanofilms and graphene-based com-posites; and 3-D nanomaterials have three dimensions outside the nanoscale,e.g. heterogenous nanostructures like mesoporous carbonbased compositesand nanostructured networks.The DEPA report (Environmental Project No. 1206, 2007) distinguishes be-tween six types of nanomaterials based on the shape characteristics of the ma-terials:••••••Nanoparticles with all three dimensions in the nanoscale range (eg, TiO2nanoparticles);Nanofibres and tubes with at least two dimensions in the nanoscale rangeand an aspect ratio of more than 3 (primarily carbon nanotubes);Nanostructured surfaces (protrusions or grooves in the nanoscale range);Nanofilm (coatings with layers thinner than 100 nm e.g. cured film onglass);Nano Flakes with at least one dimension in the nanoscale range (eg nano-clay (silicate) and the materials used in semiconductor elements);Nanoporous structures with pore sizes in the nanoscale range (such asceramic materials used as catalysts).
The most commonly available engineered nanomaterials can be organised intofour types (EPA, 2007):
14
Carbonbased materialsCarbonbased materials are composed mainly of carbon and commonlyshaped as hollow spheres, ellipsoids, or tubes. Spherical and ellipsoidal carbonnanomaterials are referred to as fullerenes, while cylindrical ones are callednanotubes. These particles have many potential applications, including im-proved films and coatings, stronger and lighter materials, and applications inelectronics.Metal-based materialsThese nanomaterials include quantum dots, nanogold, nanosilver, zero valentiron and metal oxides, such as titanium dioxide, and cerium oxide. A quan-tum dot is a closely packed semiconductor crystal comprised of hundreds orthousands of atoms, and whose size is on the order of a few nanometers to afew hundred nanometers. Changing the size of quantum dots changes theiroptical properties.DendrimersThese nanomaterials are nanosized polymers built from branched units. Thesurface of a dendrimer has numerous chain ends, which can be tailored toperform specific chemical functions. This property could also be useful forcatalysis. Also, because three-dimensional dendrimers contain interior cavitiesinto which other molecules could be placed, they may be useful for drug de-livery.CompositesComposites combine nanoparticles with other nanoparticles or with larger,bulk-type materials. Nanoparticles, such as nanosized clays, are already beingadded to products ranging from auto parts to packaging materials, to enhancemechanical, thermal, barrier, and flame-retardant properties.1.2Nanomaterials in consumer products
Nanomaterials which are already widely used in various consumer productsand therefore also are focus for research into environmental, health and safetyaspects of the materials include:
Carbon tubes and fullerenes. Carbon materials have a wide range of uses,ranging from composites for use in vehicles and sports equipment, to in-tegrated circuits for electronic components.Cerium dioxide. Nano cerium is being investigated for uses ranging fromdrug delivery to automobile catalytic converters.Currently a major use insome countries is as a diesel fuel additive to reduce exhaust particulatesand increase fuel mileage.Titanium dioxide. Nano titanium dioxide is currently used in manyproducts. Depending on the type of particle, it may be found in sun-screens, cosmetics, food additives and paints and coatings. It is also beinginvestigated for use in removing contaminants from drinking water.Silicium dioxide. Silicium dioxide is like titanium dioxide used in manyproducts including sunscreens, cosmetics, paints and cement. Siliciumdioxide is also used in the food industry.
15
Silver. Nanosilver has long been known for its antimicrobial properties.Nanosilver is being incorporated into textiles and other materials toeliminate bacteria and odor from clothing, food packaging, and otheritems where antimicrobial properties are desirable.Iron. While nano-scale iron it being investigated for many uses, including“smart fluids” for uses such as optics polishing and as better-absorbediron nutrient supplement, one of its more-prominent current applicationsis for remediation of polluted groundwater and soil.Zinc oxide. Zinc oxide is very UV-stable and is used as sunscreen incosmetic products and UV-stabiliser in plastics. Zinc oxide alsoexhibitsanti-bacterial properties which are utilised in pharmaceutical applications.Nanoclay. Nanoclay (aluminium silicon oxide) is used as additive for re-inforced plastics improving bot mechanical and thermal properties as wellas barrier characteristics. It is used in food packaging where it reduces thepermeation rate of oxygen through the packaging material and more re-cently it has also been used as synergist flame retardant to substitute thehalogen-containing flame retardants.
Other types of nanomaterials are gold nanoparticles and dendrimers, but theyare mainly explored in relation to medical and other more specialised applica-tions, although patents are already filed for the application of dendrimers invarious cosmetic products.Examples of consumer products (PEN, 2011) that use nanomaterials are:
Health and Fitness: toothpaste, toothbrush, tennis racket, air filter,sunscreen, antibacterial socks, cometics, waste and stain resistantpants, golf clubs, wound dressings, pregnancy tests, bath and sportstowelsElectronics and Computers: computer displays, computer hardware,gamesHome and Garden: paint, antimicrobial pillows, stain resistant cush-ions, humidifiers, cleaners, fabric softernersFood and Beverage: non-stick coatings, antimicrobial refrigerator, ca-nola oil, food storage containers, packagingOther: coatings, lubricants
1.3
Special characteristics of nanomaterials vs. bulk materials
The special characteristics and properties of nanomaterials are largely attrib-uted to the small size and the very large surface area to volume ratio. Thismakes a large fraction of the atoms available on the surface and results inmore surface-dependent material properties which again may enhance ormodify the properties of the bulk material.It should however be stressed that many conventional bulk chemicals withvarious applications in e.g. consumer products, foodstuffs and constructionmaterials also contain a naturally occurring nanosized fraction. This is thecase for titanium dioxide, silicium dioxide and clays. Other examples include
16
products containing nanosilver particles, which have been commercially avail-able for over 100 years, and have been used in applications as pigments, pho-tographics, wound treatment, conductive/antistatic composites, catalysts andas biocides Nowacket al.,2011).Nanomaterials in general are known to have many novel properties that arealready utilised or explored for use in different products and technologies andthat allow new areas of application of these materials. These properties in-clude mechanical, thermal, biological, optical and chemical properties, whichmay also affect the potential exposure to the materials, as well as the healthend environmental effects from that exposure. Information on potential haz-ards to health and environment is therefore urgently needed.A key challenge in relation to characterisation of the materials is that the dif-ferent nanomaterials exist in various forms, sizes and shapes and cannot bedescribed by a unique set of parameters. Their small sizes, complex struc-tures, potential property changes during synthesis and use, sensitivity to andinteraction with the surrounding environment also add to the challenge ofcharacterization and providing sufficient information for assessing potentialrisks of the nanomaterials. Furthermore information available in the literatureis often very scarce and not always reported in a form relevant for risk assess-ment.Key physical-chemical parameters (list of endpoints) to take into account,when testing specific manufactured nanomaterials for human health and envi-ronmental safety within phase one of the OECD testing programme include(OECD, 2010):Agglomeration and/or aggregationWater solubility / DispersabilityCrystalline phaseDustinessCrystallite sizeParticle size distribution - dry and in relevant mediaSpecific surface areaZeta potential (surface charge)Surface chemistry (where appropriate)Photocatalytic activityPour densityPorosityOctanol-water partition coefficient, where relevantRedox potential
17
Radical formation potentialOther relevant physical-chemical properties and material characterisa-tion information (where available)
Further studies investigating the relation between these parameters andthe toxicity of nanomaterials are needed, and will need to be addressed atsome point in the evaluation and risk assessment of nanomaterials.The current EU legislative framework for chemicals covers in principlethe potential health, safety and environmental risks posed by nanomateri-als. However, there is also a recognised need to modify this legislation, inorder to reflect the specific properties of nanomaterials, and the need formore elaborate characterisation of the nanomaterials compared to theconventional bulk form to include e.g. the specific surface properties. Theexisting test requitements for bulk chemicals may also not be adequate inall areas of toxicity, and the same is the situation with regard to classifica-tion and labelling of substances and mixtures and thereby also toxicity-based thresholds based on these criteria.REACH provides the overarching legislation applying to the manufacture,placing on the market, and use of substances on their own, in preparationsor in articles. The current view from the Commission is, that the legisla-tion in place to a large extent covers risks in relation to nanomaterials, andthat the risks can be dealt with under the current legislative framework in-cluding REACH. However modification is expected for example with re-gard to thresholds used in some legislation and with regard to testingmethods, test guidelines and risk assessment of nanomaterials.1.41.4.1Use of nanomaterials in DenmarkIndustry and products
There is no single source of information that provides an overview of the useof nanomaterials and products in Denmark or in the EU for that matter.Pieces of information are however available.Recently, the Nanowerk published an online Company & Labs directory with4,196 links to labs, associations, networks and companies. This directory in-cludes only companies and labs that work with and/or commercialisenanotechnology and/or nanomaterials and does not include entities that onlyhave “nano” in their name.According to Nanowerk there are 18 commercial companies in Denmark in-volved in various fields of nanotechnology. The majority are relatively newand very specialised companies and some have emerged from university re-search. One or two do not seem to be operational anymore in December 2010based on their homepage information. The list of companies is presented inAnnex 1.1
1
http://www.nanowerk.com/nanotechnology/research/nanotechnology_links.php
18
1.4.2
Results from selected Nordic surveys
Conclusions regarding consumer products from three recent surveys fromDenmark and Norway on products containing nanomaterials or based onnanotechnology are presented in this section.Survey on production and application of nanomaterials in Danish industryIn 2007 DEPA published a survey of the nanomaterials being applied andproduced within Danish industry, how these materials are handled in proc-esses and how the waste from these processes and products is disposed (Envi-ronmental Project No. 1206, 2007). It was found that a total of 24 companiesworked with nanomaterials of which 16 companies worked with nanoparticles,nanofibres or nanoflakes within nine different industrial areas: Paints & inks,coatings, cosmetics, pharma & biotech, optics, sensors, catalysts, concrete andtextile. More than half of the companies (i.e. 9) worked on a R&D level orused nanomaterials in their processes on a very small scale (<1 kg per year),whereas a few companies (i.e. 7) within the field of paint & ink, concrete, tex-tile or cosmetics industries worked with >100 kg nanoparticles per year. Mostused nanomaterials were metal oxides, polymers, silica and carbon blacknanoparticles which had been obtained from foreign suppliers in powder orsuspended form. Only one company reported the nanomaterial to be the end-product, and this product was produced on a very small scale (<1 kg peryear).Based on the survey and responses from 24 Danish companies working withnanomaterials, it was concluded, that with the exception of textiles, all prod-ucts (made in Denmark) on the market containing nanomaterials have a con-tent of nanomaterials above 0.1%. The nanoparticle content was 0.1 - 10 % insunscreen and coatings and more than 1 % in paint and concrete. It was fur-ther concluded that the pace for new industrial applications of nanomaterialsseemed less rapid than previously predicted and that new applications ofnanomaterials are restricted to small scale or R&D use, and have not yetreached a scale of production requiring the use of more than 1 kg of nanoma-terial per year.Table 1 summarizes the nanoparticulate materials for which the specificchemistry was identified (Environmental Project No. 1206, 2007).Table 1 Specifically identified nanparticulate materialsNanoparticulateCas NoIndustrial area (sizes)materialTiO2(rutil + anatase) 13463-67-Cosmetics (10-20 nm)7Paints & inks (nano/microparticles)TiO2(anatase)1317-70-0Coatings (9-25 nm)Fe2O31309-37-1Paints & inks (nano/microparticles)Carbon black7440-44-0 Paints & inks (nanoparticles)Textiles (nanoparticles)Silica (amorphous)7631-86-9Paints & inks (10-20 nm)Coatings (10-25 nm)Concrete (100-200 nm)ZnO1314-13-2Cosmetics (nano/microparticles)Ag7440-22-4 Coatings (nanoparticles)Textiles (nanoparticles)Cu7440-50-8 Coatings (nanoparticles)Scale> 1 ton per year> 100 tons per year< 1 kg per year> 100 tons per year> 1 ton per year> 10 tons per year> 1 ton per yearkg per yearNot reportedNot reported< 1 kg per year< 1 kg per year< 1 kg per year
19
The particle size of the used materials varies from approximately 10 nm andmore and in the case of metaloxide (pigments in the paints & ink industry),silica (concrete industry) and zinc oxide (cosmetics) the medium particulateparticle size is >> 100 nm. The companies estimate particle size distributionof the used materials is to be so broad that an unknown fraction of the parti-cles were falls within the usual definition of nanoparticles.Commercialised nanoproducts in DenmarkIn 2007, DEPA initiated a survey in order to identify consumer productsavailable to the Danish consumer (Consumer Survey No. 81, 2007). The sur-vey was based on interviews and questionnaires submitted to stakeholders inDenmark, internet searches and follow-up on search results of consumerproducts in the Consumer Product Inventory maintained by the Project ofEmerging Nanotechnologies at the Woodrow Wilson Center in Washington,DC, USA.As there is no legal requirement for producers or importers of products to de-clare the content of nanomaterials, it is not possible to be certain, that a pro-ducer or importer who uses the prefix ‘nano’ in association with a product arereferring to a content of nanoparticles, or if a nanomaterial is formed duringuse or whether it is the technology behind the product that is ‘nano’ (Con-sumer Survey No. 81, 2007).The survey found that 243 products based on a nanomaterial were availableon the Danish consumer market. The searches for Danish importers and dis-tributors of products in the Woodrow Wilson database and Danish web shopsselling these articles showed that two out of three products registered in theU.S.A. in general are for sale in Denmark (Consumer Survey No. 81, 2007).The report further concludes, that more than two thirds of the products onthe Danish market (i.e. 154 products) – are various liquid products, partly forsurface treatment of a great number of materials such as glass, concrete, metal(especially car maintenance) glass fibres and textiles, and partly for skin pro-tection products, especially sun lotions. The remaining products are in par-ticular sporting goods- and clothing, which account for 60 out of the 99 re-maining products (Consumer Survey No. 81, 2007).More than half of the consumer products on the Danish market are productsfrom Europe. Out of the 135 European products on the Danish market, al-most 100 come from Germany. The remaining products originate fromUnited Kingdom, Finland and France. Three products are sun lotions formu-lated in Denmark. In 202 out of the 243 products it was not possible to iden-tify the nanomaterial in the product. Of the 41 known nanomaterials, half ofthem were found in cosmetic products (six products with zinc oxide and 13with titanium dioxide), 10 with antibacterial silver in textiles and home appli-ances, and 12 with carbon tubes or balls (seven with carbon tubes in sportinggoods and five with fullerenes in cosmetics) (Consumer Survey No. 81,2007).As part of the survey it was found, that a considerable part of the consumerproducts are sold in web shops in Denmark and abroad, especially productsfor surface treatment within the product types `Car care products and acces-sories’, ‘Home and gardening’ and ‘Personal care and sports equipment’, buta smaller and increasing part is found in ordinary shops. A group of paintscontain ‘carbon black’ (20-100 nm) as colouring agent or silica (down to
20
approx. 10 nm) as thickening agent. Both these materials have been used for anumber of years, but are only now recognized as nanomaterials. In the DanishProduct Register a great number of individual products are registered withcarbon black (approx. 9,500) or silicium dioxide (approx. 15,500). The regis-trations do however not include information about whether these substancesinclude particles in the nanoscale. The registered amounts used in paints are483 tons carbon black and 622 tons silicium dioxide. The individual productscontaining these materials have not been further analysed. (Consumer SurveyNo. 81, 2007).With regard to potential exposure of consumers the survey concludes that inmost products containing nanomaterials on the Danish market the nanomate-rials are suspended in liquid and that these products constitute the greatestlikelihood of exposure of the consumer. These products include products forsurface treatment and cosmetics. No products with free nanoparticles wereidentified. (Consumer Survey No. 81, 2007).Survey on production, import and use of nanomaterials in NorwayIn the survey carried out in 2010 based on questionnaires sent to companiesexpected to produce, import or use nanomaterials, 27 out of 162 respondedpositively. Nanomaterials were primarily titanium dioxide, polymers, carbonnanotubes and carbon nanofibres mostly in powder form. Other forms in-cluded suspensions, composite materials, and films. Particle size was reportedto be in the range of 20 to 100 nm for all types of powder (Norwegian LabourInspection Authority, 2010).The Norwegian Product Register has in mid 2009 started voluntary registra-tion of products containing nanomaterials. Registration covers in general allproducts with a hazard classification and produced or imported in volumes of100 kg or more. Cosmetics are not included. By 20 June 2010 19 productscontaining nanomaterials were registered of which 13 were consumer prod-ucts. Typical products were paints and lacquers, care care products, productsfor impregnation and windscreen wash (Norwegian Labour Inspection Au-thority, 2010).Use of nanomaterials in Sweden in 2008 - analysis and prognosisThe Swedish Chemicals Agency has published a survey on the use of nano-materials in Sweden in 2008 (KemI, 2009). According to this survey, thenanomaterials in Swedish nanoproducts can be categorised as 7 % ceramicmaterials, 13 % carbon-based, 7 % metals, and 5 % polmers in terms of thenumber of products. It is also stated in the report that it is difficult to get atrue picture of nanoproducts that are on the market. This is partly due tothe fact, that in most cases it is not obvious from the product information,that the product contains a nanomaterial, nor what material it is, if they do.Another problem is, that consumers are purchasing the nanoproducts di-rectly on the Internet.
An overview from an English summary of the report of examples ofnanomaterials in products on the Swedish market is shown inTable 2.
21
Table 2 Examples of nanomaterials contained in articles on the Swedish marketARTICLE ON SWEDISH MARKETPROBABLE NANOMATERIALCoatings for protection and self-cleaning effecttitanium dioxide, silicon dioxide/glass,on cars, tiles, stone, glass, textilespolymersPaints and plastichyperbranched polymersRacketscarbon nanotubes, silicon dioxide compositeBicyclescarbon nanotube composite, aluminiumSkis and ice-hockey sticksepoxy carbon nanotube compositeTennis ballsnanoclay compositeCar componentspolymer-clay compositesFiller in car tyres, black inkcarbon blackFilter for treatment of intake air to enginesnanofibres of polymersSocks, soles, dressingssilver nanofilamentClothingfluorinated fibresWater repellent on textilesdendrimers, hydrophobicSunscreentitanium dioxide, zinc oxideToothpastehydroxyapatitePaper chemicalssilicon dioxideElectron microscopygold particles
The report estimates, thatfew new nanoproducts will be introduced inSweden during the next five years or so, while further testing anddevelopment of legislation takes place.Other reportsA number of other reports from the Nordic countries discussing more generalaspects and effects of nanomaterials in e.g. the work environment areavailable, but are not referred to here, as they do not include market surveys.1.5Industry survey
For the nanomaterials selected for this investigation (Chapter 2, Table 3), alimited survey on the industrial use in Denmark has been conducted. The ob-jective of this survey was - if possible - to confirm the use of the nanomaterialsin question in Denmark, and to develop a rough estimate of the consumption.The survey was carried out among identified actors dominating the marketsfor the selected nanomaterials and their typical applications. The relevantactors were asked about the use of the nanomaterials. The focus has been onobtaining information for the most dominant field of application and not tocover all different use areas.The outcome of the survey can be summarised as follows:Titanumdioxide, nanoclay and silicium dioxide are all materials usedin most significant quantities in Denmark.The use of nanosilver has not been confirmed, but indications existthat some product/brands may contain nanosilver.The use of cerium dioxide has not been confirmed either. It is notused by leading marked actors in Denmark.
No information was available on fullerenes and zero-valent iron.
22
22.1
Nanomaterials surveySelection of nanomaterials for the survey
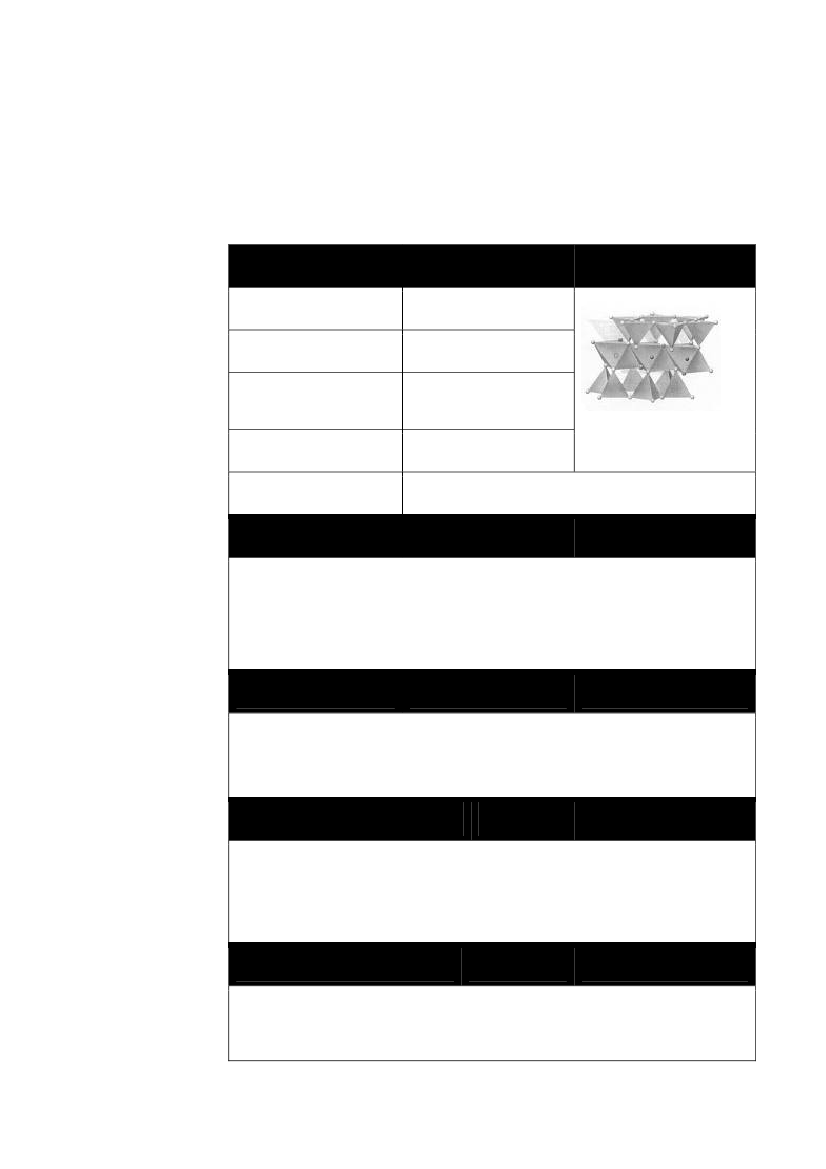
The nanomaterials selected for review in this project are shown in Table 3.The selected materials are all used in consumer products and are relevant tointernational discussions on the exposure and effects for humans and the en-vironment. Furthermore it was important, that different application scenarioswere involved. In addition, the following criteria were used for selection ofindividual materials:1. Application Volumes2. Potential human exposure3. Potential direct discharge to the environment4. Expected biological effect (human and / or environment), persistenceor bioaccumulationTable 3 Selected nanomaterials, use and criteria for selectionApplication –examples of productsBasis forMaterialsand functionselection(criteria asshown above)TitaniumdioxideCerium dioxideFullerenes(carbon balls)SilverZero-valent ironSunscreen, paint (catalyst, self-cleaning surfaces)Fuel additive (catalyst)Motor oil, cosmetics (lubricant, anti-oxidant)Textiles, electrical appliances(biocide)Remediation of soil andgroundwater contaminations(reactant, catalyst)Surface treatments, paints,(hydrophobic surfaces)Plastic materials (lower oxygendiffusion), cement1, 2, 3, 41, 2, 32, 31, 2, 3, 43, 4
Silicium dioxideNano-clay
1, 2, 3, 41, 2
Nanotubes are not considered, as they are already covered by another projectwith a similar focus.With the material selection shown in Table 3, it is expected, that the majorhealth and environmental problems specific for nanomaterials can be identi-fied in relation to the intended application and the environmental fate of thenanomaterils.The aim is to provide an overview of the existing knowledge, to identify areasin which the knowledge can be generalised, and to summarize the presentstate-of-knowledge in short and focused human health and environmentprofiles for each material.
23
To focus the characterisation, it is the pure form of the nanomaterials that isdiscussed in the following, thereby ignoring that various kinds of doping andcoatings might exists. Furthermore, the overview of the key characteristics isprimarily based on the form of the nanomaterials that is commerciallyavailable.2.2Nanomaterials profiles
In chapter 2-7 a profile for each of the selected materials will be developed.For each material the focus has been on the general characteristics and manu-facturing of the nanomaterials, their current uses (mainly focussed at con-sumer products), and hazard profiles (ecotoxicity and human toxicity).2.2.1Manufacturing and applications
The manufacturing processes and applications are described based on a litera-ture review, and the available information from the small industry survey iaalso included. Furthermore, an updated review of the commercially availableconsumer products in Denmark containing the selected nanomaterials is in-cluded in this report based on the methodology described in Consumer Pro-ject No. 81 (2007). Outset is taken in the online “Nanotechnology ConsumerProducts Inventory” maintained by the Project of Emerging Nanotechnolo-gies at the Woodrow Wilson International Center for Scholars. TheNanotechnology Consumer Products Inventory was launched in 2005 withthe inclusion of 54 products – a number that one year later had increased to356 products. In the years 2007-2009, the number of products listed in theinventory continued to increase (580 products in 2007, 803 products in 2008,and 1015 products in 2009). In the most recent update in March 2011, a totalof 1317 products were listed as commercially available worldwide from a widevariety of producers and countries (Woodrow Wilson Inventory, 2011). Forproducts to be included in the inventory it has to fulfil mainly the followingconditions: The products can be purchased directly by the consumers or iden-tified by the producer or another source as based on nanotechnology and theinformation about nanomaterials in the product seems probable.The database divides the products in a number of categories: Appliances(heating, cooling and air; large kitchen appliances, air cleaners and aircondition devices, domestic appliances, bio-up and textile protectionproducts), Automotive (exterior) maintenance and accessories, Goods forchildren (basics; toys and games), Electronics and computers (audio; camerasand film, computer hardware; display; mobile devices and communications,television; video), Foodstuffs (cooking, foodstuffs, storage, dietarysupplement), Health and fitness (clothing, cosmetics, filtration, personal care,sporting goods, sun screen), Home and garden (cleaning, constructionmaterials, home furnishings; luxury products; paint), and Surface treatment(overlapping several groups).In order to identify products that contain nanomaterials, and which arecommercially available in Denmark, it was investigated if the productsregistered in the database, are also marketed in Denmark or may be availablethrough a web shop. This was done for the 2009 data since the most recentupdate coincide with the termination of the present project.It should be noted however that, as there is no legal requirement to producersor importers of products to declare the content of nanomaterials, it is not pos-
24
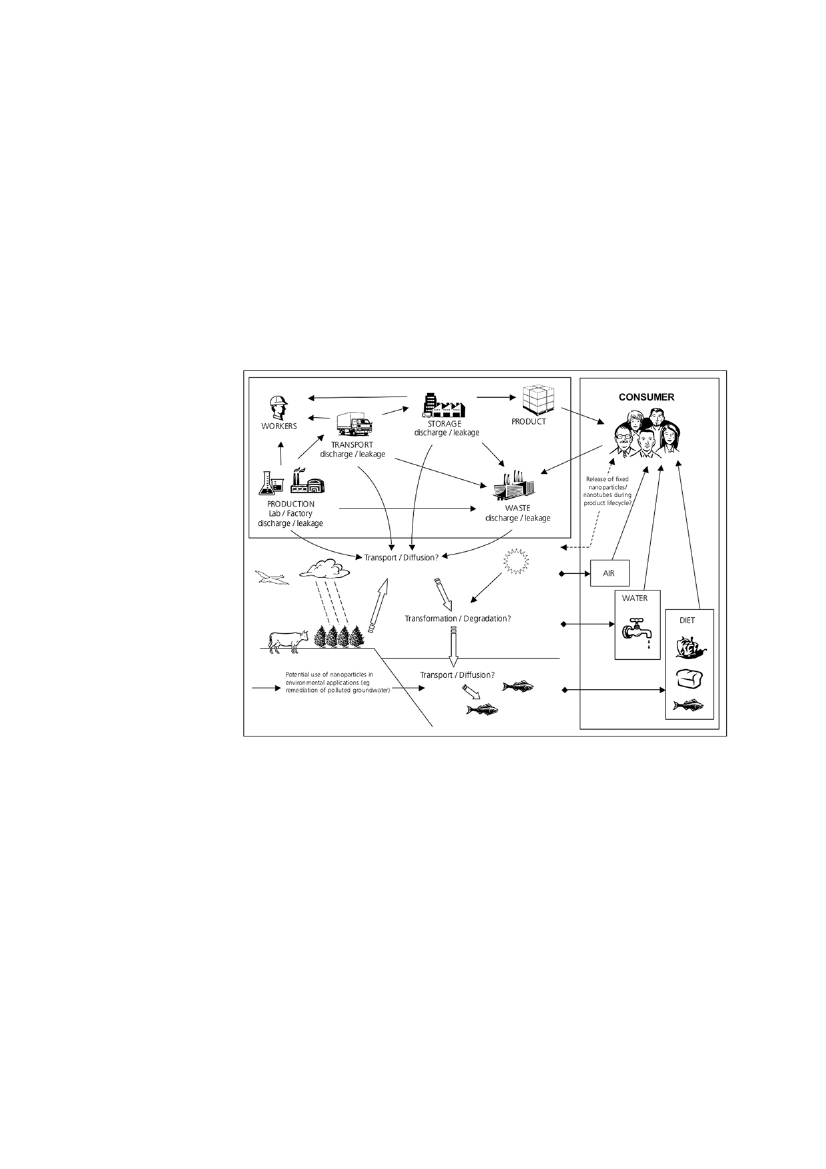
nanomaterials cannot be estimated with reasonable certainty. This is evenvalid for materials like titaniumdioxide, which is a high-production volumechemical (i.e. produced in more than 1000 tons/year) mainly used as a pig-ment in its bulk form. However, data on how much of the total amount of ti-taniumdioxide produced is in the nano-scale range are not publically available.This is due to the fact that no specific inventories for nanomaterials exist.For the environment, less than 15 scientific studies have been published onmodeling approaches to quantify environmental concentrations (e.g.Arvidssonet al.,2011; Blaseret al.,2008; Gotteschalket al.,2010a,b; Mueller& Nowack, 2008). None of these have been validated by actual monitoringdata. Less than 10 scientific studies report on measured concentrations ofnanomaterials in environmental matrices and in most cases the studies arecarried out under controlled conditions simulating environmental exposures(e.g. Kaegiet al.,2008; Kaegiet al.,2010; Farréet al.,2010)Figure 2Plausible exposure routes for nanomaterials (from Stone et al, 2010)
2.2.4
Risk profiles
The findings with regard to ecotoxicity and human toxicity, and informationabout relevant exposures are used to develop risk profiles for each of theselected nanomaterials. A summary sheet at the end of each chapterdescribing a nanomaterial, summarises the findings and are intended to formthe basis for information material in the form of short data sheets in Danishfor each nanomaterial.As evidenced by the ecotoxicological and toxicological profiles, large datagaps can be found within effects assessment, however, it may be claimed thateven data gaps can be found within exposure assessment at present. Theseuncertainties do for most nanomaterials hamper quantitative risk assessmentslike those traditionally made for industrial chemicals. Therefore, this reportdoes not attempt to make these kinds of risk estimations, but merely providedata for information purposes.
26
33.1
Fullerenes - C60General characteristics
Fullerenes consist of a family of soccer-ball shaped molecules with 60 or morecarbon atoms arranged in closed spherical and elliptic structures as each car-bon atom bonded to three others in pentagonal and hexagonal rings. The sizeof a fullerene is approximately 1 nm. C60has long been regarded as the posterchild of nanotechnology, but the fullerenes furthermore consists of the C70, C76and C84(Health and Safety Executive, 2004). Fullerenes can furthermore con-sist of a number of layers which are called onions (Terrones & Terrones,2003). C60-fullerenes are extremely stable and are extremely temperature andpressure resistant. Fullerenes have a tendency to agglomerate and form clus-ters of crystals, termed nanoC60or nC60. Although normally considered to bean insoluble material, C60can be surface modified making it more water solu-ble and thereby provide them with a great variation in their physico-chemicalproperties as well as biological activity (Stoneet al.2010). Modification canbe through the attachment of e.g. hydrophilic groups, peptides, or carbonhy-drates which changes the properties of C60(Sayeset al.2004, EuropeanCommission, 2005).3.2Manufacturing processes
Different methods exists to produce fullerenes, but most large scale manufac-turers use a combustion process, which uses hydrocarbons as a raw materialto produce C60. Toluene is fed together with oxygen to a low-pressure com-bustion chamber. The flame produces fullerene-enriched soot, which is ex-tracted and filtered (Takearaet al.,2004). Fullerenes are then extracted fromthe soot by solvents (e.g. chlorobenzene and toluene) in a tank where the in-soluble soot settle to the bottom while C60are dissolved in the solution. Puri-fied fullerenes are collected after the solvent has evaporated and appear as ablack powder. The separation of single size fullerenes (C60,C70) and the degreeof purity determine the price of the final product.3.33.3.1UsesMain applications
C60has been applied in a number of different consumer products such assports gear (badminton- and tennis rackets), cosmetics and personal careproducts (anti-aging, eyeliner, skin creams, etc.) and also lubricants (motoroil) (Francoet al.2007, Consumer Survey No. 81, 2007). Other current usesinclude energy applications (such as fuel cells, solar cells and batteries), cata-lysts, polymer modifications and targeted drug delivery systems (Aschbergeret al.2010). In total five products have been identified to be commerciallyavailable on the Danish marked. While C60-containing cosmetics and personalcare products can be bought online (Francoet al.2007, Consumer SurveyNo. 81, 2007), only products related to sports gear have been identified onthe Danish market. In these products C60is added to strengthen the structureof e.g. tennis rackets where the C60-fullerenes are dispersed in a resin between
27
carbon fibers. The C60content of e.g. sportsgear and lubricants is unknown.For patents filed in US in regard to cosmetics, concentrations between 0.05and 6 % are reported. Concentrations used are the highest in eyeliner andmascara with 6% and 5%, respectively (Boxallet al.2008). In lubricants,fullerene soot containing approximately 3.2 % fullerenes is added to improvesliding between metal surfaces. With a soot content of 9.4 %, the concentra-tion of C60is estimated at about 3 g/kg. The fullerene molecules are expectedto be partly free during use and unintended release should therefore be con-sidered in an exposure assessment. (Francoet al.,2007).The production and use of fullerenes is assumed to be limited at present, butexpected to grow significantly over the next decade. In Japan a large scaleproduction plant has been opened recently with a production capacity of 40tons per year. (Aitken e al 2006 and Fujitani et al 2008 in Aschberger et al2009).3.3.2Results from industry survey
No information on fullerenes has been obtained.3.4Eco-toxicological profile
For the following overview of the ecotoxicological profile of C60-fullerenes itshould be stressed that only a few ecotoxicological studies exist and only apart of these are aimed at the base-set organisms (fish, crustacean and algae)required for doing effects assessment according to REACH. Even fewer stud-ies report the results in terms of the endpoints and values listed in InformationRequirements of the REACH (e.g., LC50, EC50, NOEC, LOEC) (ECHA,2008).The testing of C60in ecotoxicological tests is difficult due to the very low wa-ter solubility of the compound. This has lead to the use of organic solvents,which has been shown to influence the ecotoxic response. Thus, studies havedemonstrated that the degradation of tetrahydrofuran (THF), used as a sol-vent in many of the early ecotoxicity studies on C60, may in fact be the causeof the toxicity observed (Stone et la. 2010). In general, less adverse effects areobserved when using water-stirred or sonicated C60compared to C60tested inthe presence of tetrahydrofuran or dimethylsulfoxide as solvents. For instance,a number of studies observed no effect on the survival of larval zebrafish andfathead minnow after exposure to stirred and sonicated C60, whereas increasedmortality and elevated lipid peroxidation was found after exposure to THF-C60(Henryet al.2007, Zhuet al.2006a; Oberdörsteret al.2006). Evidencefrom studies on Daphnia magna also shows a large difference between theecotoxicity of water-stirred and THF- C60(Lovern and Klaper 2006, Lovernet al.2007,et al.Zhuet al.2006). These interactions lead Stoneet al.(2010)to the conclusions that ecotoxicological studies carried out with tetrahydrofu-ran do not have a high credibility.In C60-suspensions prepared by long-term stirring, Zhuet al.(2008) observedno mortality or unusual behaviours of juvenile carp (Carassius auratus) after32 days of exposure to between 0.04–1.0 mg/L. However, a significantly re-duction in the mean total length was observed after 32 d exposure to 0.2 mg/Lof C60and a significantly reduced body weight at 1.0 mg/ l. No detectable ef-fects were observed after exposure to 0.04 mg/L for 32 days. This might cor-respond to a NOEC of 0.04 mg/L and a LOEC of 0.2 mg/L for the length of
28
juvenile carp and a LOEC of 1.0 mg/L for the body weight after exposure toC60in water for 32 days.For crustaceans, the 48 h lethality study by Lovern and Klaper (2006) re-ported on a great variation in mortality in Daphnia magna, but a LC50, 48hof 7.9 mg/L for water-sonicated C60could be established LOEC and NOECwere reported to be 0.5 mg/L and 0.2 mg/L, respectively.In regard to survival and reproductive endpoints a significant reduction in thenumber of offspring after 21 days and delays in moulting of the carapace wasobserved by Oberdörsteret al.(2006) after exposing Daphnia magna to 2.5mg/L water-stirred C60. An increased cumulative mortality and significant de-lay in moulting and reduced offspring was reported as well after exposure to1-5 mg/L for 21 days (Oberdörsteret al.2006).For earthworms no significant mortality was found after consuming dry foodspiked with 99.5% C60concentrations of 1 g/kg dry food for up to 28 days(Scott-Fordsmandet al.,2008). Johansenet al.(2008) found that exposure to50 mg/kg to 99.5% C60aggregates caused a 60% inhibition of the number ofbacterial colony-forming units CFUs in clay loam soil 3 hours after incorpora-tion.No studies on bioaccumulation of fullerenes have been reported in the litera-ture. No studies on the degradability of fullerenes have been found in the lit-erature. The cage-like structure of C60suggests very low biological degradabil-ity, however functionalisation (e.g. hydroxylation) may alter the degradabilitybehaviour significantly. It has been suggested that C60can be oxidised toC60fullerol through both abiotic- and biotic-mediated means (Schreineret al.2009). Two white rot basidiomycete fungi (Phlebia tremellosa and Trametesversicolor) has again been demonstrated by Schreineret al.(2009) to metabo-lize and degrade C60-fullerol to CO2after 32 weeks of decay, with minoramounts of the fullerol carbon incorporated into lipid biomass.Finally, it should be mentioned that Baunet al.(2008) found that the pres-ence of C60in toxicity tests increase the toxicity of phenanthrene. It was fur-thermore found that the uptake of phenanthrene in D. magna was faster in thepresence of C60. A 1.7 times higher steady-state concentration was reached inthe animals. However, a very fast clearance took place when animals weretransferred to clean water resulting in no accumulation of phenanthrene(Baunet al.2008).3.5Toxicological profile
Bothin vitroandin vivostudies have been performed on fullerenes, but mostof the studies have some limitations. A number of the toxicological studieswith fullerenes are relatively old, and therefore they do not focus on the“nano” dimension of fullerenes. None of the studies are performed accordingto guidelines (e.g. OECD). Different dispersants used to enhance dispersionand to minimise cluster/crystal size can also influence the toxicity. The factthat a number of fullerene derivates are available, with different number ofcarbon atoms (e.g. C60or C70), and different surface modifications used torender fullerenes water soluble can also complicate the toxicological evalua-tion of fullerenes.
29
A particular focus of the more recent studies has been to determine the anti-oxidant properties of fullerenes, and how to improve their dispersion withinaqueous suspensions. The most relevant of the studies for risk assessment aresummarised below and quoted from Stoneet al.(2010) and Aschbergeret al.(2009).3.5.1ADME studies
Determining the kinetics of fullerenes within the body, subsequent to expo-sure (via the lungs, gut and skin) is necessary to identify potential targets offullerene toxicity, and thereby direct relevantin vitroassessments of their tox-icity at particular target sites. However, only few studies provide evidence forthe absorption of fullerenes into the blood from their exposure site.AbsorptionInhalationIn a study by Bakeret al.(2008) nano and microparticulete forms of fullere-nes were not detected in blood following inhalation by rats, suggesting thatthey do not translocate from their exposure site. A half life of 26 days forfullerenes nanoparticles was determined which is similar to microparticles (29days) suggesting that similar elimination processes are involved during theremoval from the lungs. However, the pulmonary deposition fraction was 50% higher for the nano form compared to the non-nano form. It is necessary tonote that the preparation method and therefore the form of the fullerene dis-persion could influence this data and therefore additional studies are requiredbefore this finding can be considered universal.In a rat study no translocation of C60to other organs was observed after intra-3tracheal installation (3.3 mg/kg bw) or inhalation exposure (0.12 mg/m ),supporting that there is no absorption after inhalation (Shinoharaet al.,2009)In contrast Naota et al. (2009) suggested that nano C60may be absorbed afterinstallation. However, it is unclear whether the suspension induced oedemacould have influenced the result.Oral:After oral administration to rats and mice, C60was not effectively absorbed,but instead the majority was excreted in the faeces within 48 hours. However,trace amounts of fullerene were observed in the urine, indicating that somefullerenes were able to pass through the gut wall (Yamagoet al.,1995).In a study by Folkmannet al.(2009) oxidative DNA damage was observed inliver and lung after oral exposure via gavage to C60suspended in either salineor corn oil, indicating absorption via the oral route.Dermal:In a study by Xiaet al.(2010) it was shown that pristine nanoC60 can pene-trate deep into the stratum corneum bothin vivo(tape stripping an tissue bi-opsies in weanling pigs) andin vitro(diffusion cell experiment). The absorp-tion was modulated by the solvent, in which C60was dispersed. This observa-tion underlines the importance of taken the effect of the dispersion mediuminto account in risk assessment of C60.After administration of C60dissolved in squalane (Lipo-fullerene, LF-SQ) tohuman skin biopsies at concentrations as high as 223 ppm C60in LF-SQ, C60
30
permeated into the epidermis but into the dermis, indicating that this prepara-tion of C60will not be systemically available after dermal administration (Katoet all 2009)Other studies:Severalin vitrostudies have shown that fullerenes are taken up by differentcell types often with oxidative and lethal consequences. Computer simulationhas also been used to simulate uptake, but the relevance of these studies is stillunknown. (Stoneet al.,2010)DistributionInhalation, oral, dermalThere is limited information on distribution to secondary organs, probablybecause there is no or low absorption.Other routes - injectionFollowingintraperitoneal injectioninto rats water soluble, polyalkylsulfonatedC60 were transported via blood, accumulating in liver, spleen and kidney,with evidence of toxicity at sites of accumulation (Chenet al.,1998b).Afterintravenous injectionwater solfullerenes were rapidly removed from theblood and accumulated primarily in liver, but also, presumably depending ontheir water solubility in kidney, lungs, spleen, heart and brain (Yamagoet al.,1995).Yamagoet al.(1995) investigated the distribution of 14C labelled, water solu-ble C60within rats, afterintravenous injection.Subsequent to exposure, thefullerenes were rapidly removed from the blood (only 1.6% of the adminis-tered dose remained in the blood after an hour) and accumulated within theliver, which was the primary site of localisation, although some localisationwas also evident within, for example the kidney, lungs spleen, heart and brain.In a similar study, Bullard-Dillardet al.(1996) also exposed rats via intrave-nous exposure to pristine (unmodified) and a water soluble quaternary am-monium salt-derivatised C60. Clearance of C60from the blood was againrapid. However, the clearance of quaternary ammonium salt-derivatised C60,was slower than of pristine C60due to its water soluble character. Again themajority of the unmodified particles were contained within the liver (over90%) at 120 minutes post exposure, with minimal accumulation within thespleen, lung and muscle. The water-soluble C60had a wider tissue distribu-tion, with only 50% of the administered dose evident within the liver, with theremaining dose contained in the spleen, lungs, muscle and cellular componentof blood. After 120 hours, it was apparent that the majority (95%) of unmodi-fied C60still remained within the liver, with no evidence of elimination withinurine or faeces, highlighting that the liver is a potential target for fullerene ac-cumulation and toxicity.MetabolismThe metabolism of fullerenes has been suggested to occur, following their ac-cumulation within Kupffer cells in the liver of rats (Gharbiet al.,2005). Themetabolites has not yet been identified.
31
EliminationThe elimination of fullerenes in urine (Yamagoet al.,1995) and faeces (Moriet al.2006, Yamagoet al.,1995) has been demonstrated in rat and mouse,suggesting that they may be eliminated, in part, from the body following ex-posure via a number of routes.3.5.2Short term toxicity
OralTwo studies were identified that show a very low toxicity of fullerenes subse-quent to oral exposure.No lethality, or other signs of toxicity in terms of behaviour or body weightwere evident in rats after oral exposure at a dose of 2000 mg/kg of fullerite (amixture of C60and C70), during the observation period (up to 14 days) (Moriet al.,2006). Based on this study a NOAEL of 2000 mg/kg bw/day is sug-gested.Chenet al.(1998b) demonstrated that polyalkylsulfonated (water soluble) C60showed no effects subsequent to oral exposure of rats in acute (2500 mg/kg,single administration) and as a consequence it was considered to be notacutely toxic. A NOAEL of 2500 mg/kg bw/day is suggested.In both studies only one dose was used without effect, and therefore the trueoral acute NOAEL may be higher.Inhalation/intracheal installaionA range of nanoparticles has been shown to induce pro-inflammatory effectsin the lung, however this does not seem to be the case with fullerenes.In a study by (Bakeret al.,2008) no inflammatory potential was observed inrats exposed to fullerenes following nasal inhalation at concentrations of 2.22mg/m3 (nanoparticle, 55 nm diameter) and 2.35 mg/m3 (microparticle, 0.93�m diameter) for 3 hours per day for 10 consecutive days, and following asingle intratracheal instillation at concentrations between 0.2 and 3 mg/kg C60or C60(OH)24, for a period of up to 3 months following exposure (Sayesetal.,2007).A study by Roursgaardet al.(2008) where mice were exposed via intratra-cheal instillation to doses of 0.02 to 200 �g per mouse for 24 hours it wasshown that at low concentrations (20μgper mouse), fullerols (i.e. hydroxy-lated fullerenes) may have protective, anti-inflammatory properties probablydue to the ability of fullerols to reduce ROS mediated inflammation, but athigher concentrations (200μg/mouse)they exhibit a pro-inflammatory re-sponse.DermalOnly one study on the potential skin effects of fullerenes was found. In a hu-man study Huczkoet al.(1999) used patch testing to assess the skin irritantpotential of fullerene soot within 30 volunteers (who reported irritation andallergic susceptibilities) for a 96 hour exposure time. No skin irritation wasfound.
32
Other routesIntraperitoneal exposureFollowing intraperitoneal injection in mice and rats fullerenes induced anti-genic behaviour by stimulating the generation of antibodies (Chenet al.,1998) which were also able to interact with SWCNT, (Erlangeret al.,2001).An LD50of 600 mg/kg was determined via intraperitoneal injection to ratswith water soluble, polyalkylsulfonated C60,in an acute (up to 1000 mg/kg, for24 hours) or subacute setting (up to 60 mg/kg, with daily exposures for 12consecutive days). The kidney was recognised as a primary site of fullereneelimination and toxicity (nephropathy) (Chenet al.,1998). Subsequent tointraperitoneal administration fullerenes have also been observed to accumu-late within Kupffer cells in the liver (Gharbiet al.,2005).3.5.3Irritation and corrosion
SkinThe only identified investigation was a patch test model on humans by(Huczkoet al.,1999) mentioned aboveEyeA Draize rabbit eye irritation test was performed to reveal the potential toxic-ity of fullerenes to the eye. Instillation of a fullerene soot suspension (for up to72 hours) was observed to have no toxicity within the eye (Huczkoet al.,1999).InhalationNo information has been identified on respiratory irritation.3.5.4Skin and respiratory sensitisation
No effects were seen in a human patch testing to assess the skin irritant poten-tial (Huczkoet al.,1999). No other information on skin and respiratory tractsensitisation is identified. There are indications that C60derivatives may act assensitising agents followingintraperitonealexposure (Erlangeret al.,2001).The relevance of these findings to the skin and respiratory tract has to be in-vestigated.3.5.5Repeated dose toxicity
OralNo effects were observed after subacute (50 mg/kg daily for 12 days) oral ex-posure to polyalkylsulfonated (water soluble) C60(Chenet al.,1998b). Noinformation on effects after subchronic or chronic exposure has been identi-fied.Inhalation3In a subacute inhalation study 0.12 mg/m fullerenes did not induce signifi-cant inflammation and tissue injury during the inhalation exposure period (28days) and after 3 months observation period. However, some genes associatedwith the immune system were up-regulated by C60fullerene particles. It wasconcluded that fullerenes might not have severe pulmonary toxicity (Fujitaet3al.,2009). A LOAEC of 0.12 mg/m is proposed.No inflammation was seen after 10 days nasal inhalation of 2.22 mg/m for 3hours per day (Bakeret al.,2008) and 3 months after a single intratrachealinstillation of 3 mg/kg C60or C60(OH)24(Sayeset al.,2007). No information3
33
after subchronic or chronic exposure has been identified. An acute NOAEC3of 2.22 mg/m is proposed.DermalNo information after repeated dermal exposure is identified.3.5.6Mutagenotoxicity/genotoxicity
In vitrodataSeveralin vitrogenotoxicity studies have been performed during recent yearsin different cell lines and also a fewin vivostudies. Different fullerene typeshave been tested as indicated in the text below.Gene mutation in bacteriaMutagenicity was observed in theSalmonella typhimuriumstrains TA102,T104 and YG3003 (a repair deficient strain of TA102) but only after irradia-tion with visible light, and the effect was reduced in the presence of oxygenscavengers likeβ-carotene,indicating the formation of ROS by photo activa-tion of fullerenes (Sera et. al., 1996). Highest tested concentration was 30 �gper plateNo mutagenic effect was induced within a variety ofSalmonella typhimuriumandEscherichia Colistrains by a C60/C70mixture (fullerite); up to 5000 �g perplate (Moriet al.,2006)In a study performed according to OECD guideline 471 (Shinoharaet al.,2009) with a well characterised stable suspension of C60in 0.1% carboxy-methylcellulose sodium (CMC-Na) no mutagenic effect was observed in anystrains either with or without metabolic activation and regardless of irradia-tion. The highest tested concentration was 1000 �g per plate which was thehighest achievable. Diameters of the majority of particles were < 100 nm.Lipo-fullerene (squalane and C60) was tested in 4Salmonellastrains and oneE.Colistrain according to OECD 471 up to 5000 �g per plate. No mutageniceffect was observed in this study either with or without metabolic activation.Chromosomal aberration in mammalian cellsNo numerical or structural chromosomal aberrations were induced inCHL/IU hamster lung cells (Moriet al.,2006).A dispersion of pristine C60in saline containing 0.05%Tween 80 induced aconcentration related increase of micronuclei in A549 human lung cells atconcentrations from 0.02 to 200 �g/ml. The tested suspension containedmainly agglomerates with a wide distribution range (10.5 to 12914 nm) (Tot-sukaet al.,2009).A dispersion of pristine C60in CMC-Na did not induce structural chromoso-mal aberrations in CHL/IL cells either with or without metabolic activationand irrespective of irradiation at concentration up to 200 �g/ml (Shinoharaetal.2009)The assay was claimed to be performed according to Japanese andOECD testing guidelines.Genotoxic effect in mammalian cellsFullerenes have shown to induce DNA damage within human lymphocytes ina Comet assay when exposed at concentrations ranging from 0.42 to 2100�g/L, for up to 6 hours (Dhawanet al.,.2006).
34
C60(0-200 �g/ml, 24 or 576 hours) did not increase the level of DNA strandbreaks, but there was a slight induction of FPG sensitive sites/oxidisedpurines, using the Comet assay, which could be explained by a slight induc-tion of ROS both within cells and in a cell free medium were observed when(Jacobsenet al.,2008).Mrdanovicet al.(2009) investigated the genotoxic and antigenotoxic effect offullerenol (C60(OH24) on Chinese hamster ovary cells (CHO-K1). Fullerenoldid not induce micronuclei (MN) or chromosomal aberrations (CA) at a widerange of concenrations (11 – 221 �M). Fullerenol had a protective effect (an-tigenotoxic) on both non damaged (control) and mitomycin C (MMC) dam-aged cells. A dose dependent decrease in MN frequency in non damaged cellswas found after 24 h exposure after 3 h this effect was only observed at lowerconcentrations, but MN was lower at all concentrations and time points com-pared to controls. CA frequency was lowered after both short (3h) and long(24h) treatment, but lowest after 3h (all concentrations) and at low concentra-tions after 24 h. Fullerenol had antigenotoxic effect on MMC induced MNand CA at all concentrations and time points and most pronounced on MNafter 24h treatment at low concentrations. These findings suggest an anti-oxidative effect at low fullerenol concentrations which may turn into a pro-oxidative effect at higher concentrations.In vivodataA single intragastric administration to rats of C60suspended in either saline orcorn oil induced oxidative damage measured as 8-oxodG in the liver and lungbut not in colon. There was a significant effect of corn oil itself but there wasno indication of interaction between the type of vehicle used and particle ex-posure. These data indicate that C60is absorbed from the gastrointestinal tractto blood and circulated to secondary organs resulting in oxidative DNA dam-age. (Folkmannet al.,2009).C60suspended in saline containing 0.05% Tween 80 single or multiple dosesof 0.2 mg/kg was installed intratracheally to male C57BL/6 mice or gpt deltatransgenic mice. After 3 h DNA damage was observed in the lung of maleC57BL/6 mice in the alkaline comet assay. After 24 hour this effect was de-creased, indicating DNA repair. C60also induced gpt mutations in the lungbut not in the kidney of transgenic mice the effect increased after multiple(x4) exposures. From the mutation spectrum obtained it is suggested thatoxidative DNA damage may be involved in the mutagenic effect. (Totsukaetal.,2009)A bone marrow micronucleus assay using a stable suspension in 0.1% aque-ous Tween 80 of nanosized C60was performed in ICR mice. Three doses (22,45 amd 88 mg/kg) were administered twice by gavage. There was no induc-tion of micronuclei at any doses tested. However, there was also no indicationthat the substance did reach the bone marrow (no toxicity on bone marrow orgeneral toxicity).The test was claimed to be performed according to OECD487 (Shinoharaet al.,2009).3.5.7Carcinogenicity
No information on carcinogenic effects of fullerenes has been identified.Some studies have reported anti-tumour effects of fullerenesin vivoandinvitro,depending on derivatisation, dispersion and light irradiation (Chenet al.,
35
2005, Tabataet al.,1997, Zhuet al.,2008). It would appear that fullerenescan accumulate in tumours due to hyperpermeability of tumour vasculaturewith very low toxicity to other organs. Light irradiation seems to be essentialfor tumour destructive effect to manifest.Gd@C82(OH)22 following intraperitoneal administration has been demon-strated to inhibit the growth of malignant tumours within mice, and that thiswas due to their ROS scavenging activity (Yinet al.,2008).3.5.8Reproductive toxicity
Effect on fertilityNoin vivostudies has been identifiedDevelopmental toxicityOnly onein vivomammalian study on the effects of fullerenes on the develop-ing embryo has been identified. Following intraperitoneal administration ofpolyvinylpyrrolidone solubilised C60(up to 137 mg/kg) to pregnant mice, ef-fects like abnormal enlargement of the head, tail abnormalities and dead em-bryos at the higher doses were seen as well as shrunken membrane and nar-row blood vessels of the yolk sack (Tsuchiyaet al.,1996). The NOAEL was16.7 mg/kg. This study is of limited relevance due to low number of animalsper exposure group and the unusual route of administration, using a relativelyhigh exposure dose and covering only a small part of the pregnancy period.3.5.9
Somein vitrotoxicity studies has been performed. However, since they arenot relevant for the risk assessment they will not be mentioned here.3.5.10 SummaryFullerenes exist in a variety of forms (carbon atom number, surface modifica-tions, aggregations states, etc) and it is difficult to make generalisation abouttheir toxic behaviour.So far it has been shown that one of the main factors for differences infullerene toxicity seems to be the water solubility with fullerenes of greater wa-ter solubility being less toxic.Fullerenes are assumed not to be effectively absorbed and to remain at thedeposition site (mainly lung and gut). A small amount may be absorbedthrough the gut wall. No information is available for possible dermal absorp-tion.Fullerenes seem to have a very low toxicity after oral exposure and an acuteNOAEL of 2000 mg/kg bw/day for fullerites and a sub-acute NOAEL of 50mg/kg bw/day for polyalkylsulfonated C60from a subacute toxicity study (12days) are suggested.Following exposure via the pulmonary route fullerenes were able to inducepro- or anti-inflammatory responses with the factors driving these effects stillunknown. An acute NOAEC of 2.22 mg/m3 for acute inhalation is suggestedas no inflammatory potential was seen at this concentration. In addition aLOAEC of 0.12 mg/m3 from a 28 day whole body inhalation study whereweak inflammation was observed (Fujitaet al.,2009) is suggested for derivinga DNEL for chronic exposure.
36
Identified studies suggest that fullerenes may not have an irritating potential toskin and eyes. No conclusions can be made on the sensitising properties offullerenes. Further clarification concerning an irritating and sensitising poten-tial of fullerenes is needed.No sub-chronic or chronic studies with fullerenes for any of the exposureroutes have been identified. The main effects associated with fullerene toxicityas shown byin vitroand acute toxicity studies are inflammatory and oxidativeresponses as well as anti-inflammatory and anti-oxidant properties. Furtherstudies should focus on the sublethal effects following subchronic/chronic ex-posure via relevant exposure routes at, for humans, relevant concentrationswhich have to be determined based on a better operational exposure database.These studies should investigate the chronic effects of the observed inflamma-tion. These effects could become more severe with prolonged period, therecould however also be recovery of the organism. Prolonged inflammationcould also lead to the release of factors which could induce systemic effects –however there is no indication of that yet.Identifiedin vitroandin vivomutagenicity /genotoxicity tests have reportedcontradicting results. Appropriatein vitro and in vivostudies should confirmthe presence or absence of a mutagenic effect and the conditions influencingthe test results.No information on carcinogenic effects of fullerenes has been identified andthere are indications that fullerenes may have anti-tumorigenic effects whichcould be an indication that carcinogenicity is not an endpoint of concern forfullerenes. However the identified information is not sufficient to reach thisconclusion. The conclusion on whether carcinogenicity studies are needed isdependent on more information on toxicokinetic and mutagenicity.Effects on female reproductive organs and embryos have been shown underquite artificial conditions. This endpoint should be re-evaluated once moreinformation on the absorption and subchronic/chronic effects become avail-able.One important issue to be solved is the question regarding what drives thepro- and anti-oxidative and inflammatory properties of fullerenes, to estimatethe hazard of different fullerene types and maybe allow for a grouping of themwith regard to their toxicity.Full physicochemical characterisation is essential to allow comparisons be-tween fullerene toxicity and a risk assessment should focus on the fullerenetype and concentrations to which humans are exposed.3.6Exposure scenarios
For the use of C60in sportsgear (e.g. badminton and tennis rackets) the poten-tial exposure for both humans and the environment is expected to be very lowduring the use phase of the products. This is due to the fact the fullerenes areembedded in the products e.g. in a polymer. After disposal of the products,fullerenes may be released, but since nothing is known today about release ofnanomaterials during waste management operations (e.g. recycling, landfill-ing, or incineration), exposure estimations cannot be made.
37
The highest concentrations of C60in cosmetics have been reported for eyelinerand mascara (5-6%), but C60is also used in day and night creams due to itsacclaimed effectiveness as an antioxidant (Boxallet al.,2008). In the case offacial creams used both night and day, Hansenet al.(2008) estimated aworst-case exposure of 26 �g/kg bw/d assuming a content of 0.1% of nanopar-ticles, e.g. fullerenes. Though the concentrations in eyeliner and mascara aremuch higher than this, the amounts used are far lower. After use the facialcreams etc. will be washed off and the content of fullerenes will be emitted tothe wastewater treatment plant. Although the resulting concentrations are ex-pected to be very low indeed, it should be noted that fullerenes are not readilybiodegradable (Hartmanet al.,2010 – submitted), but may end up in thesludges from the wastewater treatment plant after agglomeration and settling.Fullerenes may also be stabilized by the presence of natural organic matterand stay as colloids in the water phase (Stoneet al.,2010).The use of C60in motor oil makes exposure to both professionals and con-sumers possible. In the lubricant, soot-containing C60is mixed together withEnvironmental emissions of C60from motor oil can mainly be anticipated dur-ing filling and change of the oil. C60may also adhere to metallic componentsof the car and may therefore have to be taken into account when the car isdisposed of.Exposure to fullerenes will mainly occur via inhalation and the dermal route atthe work place.The main exposure route for consumers is expected to be the dermal route viacosmetics and personal care products and chronic exposure might be ex-pected. Oral exposure expected to be low (except for potential usage for thetreatment of cancer)The exposure via sports gear (badminton- and tennis rackets), is expected tobe negligible.Based on current use levels, the human exposure via the environment isunlikely to be at detectable levels, however this might increase in the future.For all exposure scenarios, quantitative assessment is hampered by the lack ofdata on amounts, sources, and purity of fullerenes.3.73.7.1Risk profileEnvironment
The ecotoxicity of bulk C60fullerenes is at present very poorly documented.Only a few of the ecotoxicity studies have reported the results in terms of theendpoints and values listed in Information Requirements of the REACH(ECHA, 2008).In general the acute toxicity of C60seems to be low, however a LC50, 48h-value of 7.9 mg/1 for water-sonicated C60has been reported. This would leadto a classification of C60as “Aquatic Chronic 2”. In longer-term exposurestudies (32 d) with fish a NOEC of 0.04 mg/L and a LOEC of 0.2 mg/1 forthe length of juvenile carp has been found.
38
It must be stressed, that the ecotoxicological testing of C60is difficult, due tothe very low water solubility of the compound and further method develop-ment may be needed. At present it seems clear, that ecotoxicological studiescarried out with tetrahydrofuran as a solvent, do not have a high credibilityand should not be used for hazard identification or risk assessment purposes.Based on a number of assumptions, Boxallet al.(2008) estimated regionalconcentrations of fullerenes in water to be in the order of 0.3 �g/l. While thismight be used as a first estimation of PEC, it must be stressed that this esti-mate is highly uncertain due to lack of data about the sources for fullerenes,amounts used, and the environmental transport and fate of fullerenes.As concluded by Stoneet al.(2010) the poor ecotoxicity database for fullere-nes implies that no reliable estimation of PNEC can be made. Given the qual-ity of the present PEC estimates and the lack of suitable ecotoxicity data forPNEC estimation, a quantitative risk characterization of fullerenes cannot bemade at present.3.7.2Human health
After oral exposure fullerenes showed very low toxicity and an acute NOAEL> 2000 mg/kg bw/d for fullerites and > 2500 mg/kg bw/d for polyalkylsul-fonated C60 was suggested.There is limited absorption of pristine fullerenes from the gut, and thereforeno toxicity may be expected after oral exposure. However, functionalisedfullerenes with a higher solubility may behave differently.Since no oral exposure estimates are known no quantitative risk estimates canbe performed but qualitatively very low risk via this exposure route is ex-pected.A potential risk for consumers could arise from dermal exposure to skincreams due to their direct application and potential widespread use. However,no toxicity data on dermal effects has been identified, that could be used toderive a human NOAEL. Therefore no quantitative risk assessment is possi-ble, however based on the current available information the risk is expected tobe rather low.Based on the assumption that the toxic effect of fullerenes is thresholded, ahuman indicative no-effect level (INEL) was derived (Stoneet al.,2010 andAschbergeret al.,2010) for inhalation. The key study used for this assump-tion (Bakeret al.,2008) is not a guideline study and was not performed forthe purpose of risk assessment; therefore a proper DNEL cannot be derived.A NOACH of 2.22 mg/m C60was derived based on absence of inflammationin rats. This NOAC was modified according to ECHA (2008) a correctedNOAC for the general public (24 h exposure via inhalation from air pollu-tion) and an INELchronic general public= 1.9�/m3was derived.No data have been found, that have shown adverse health effects towardsfullerenes in consumer products. Although data from the present use in con-sumer products indicate a low potential for risk towards human health thedata gaps with regard to toxicological properties as well as to exposure have tobe acknowledged. Thus complete toxiciolgical data sets on 'pure' fullerenes aswell as on the various functionalised fullerenes are still lacking. Furthermore,3
39
more precise knowledge about consumer exposure is necessary, in order to beable to make a more detailed risk assessment of fullerenes.3.8Summary sheet for C60
Nanomaterial characteristicsNameCAS numberChemical compositionAppearanceManufacturingessesproc-Fullerene
99685-96-8C60(Source: Wikipedia)Black powderControlled combustion of hydrocarbons (e.g. toluene) followed by sol-vent extraction.
Nanomaterial descriptionC60-fullerene is soccer-ball shaped molecule with 60 carbon atoms arranged in a cage closed struc-ture. The size of a fullerene is approximately 1 nm. C60-fullerenes are extremely stable and have atendency to agglomerate and form clusters of crystals when dispersed in water (nC60). C60can besurface modified making it more water soluble and thereby provide them with a great variation intheir physico-chemical properties as well as biological activity (Stoneet al.2010).ApplicationsC60has been applied in a number of different consumer products such as sports gear (badminton-and tennis rackets), cosmetics and personal care products (anti-aging, eyeliner, skin creams, etc.)and even lubricants (motor oil). The C60content of e.g. sportsgear is unknown. The content inmotor oil is estimated at 3 g/kg (Francoet al., 2007). For cosmetics contents between 0.05 and6% are reported. Concentrations used are the highest for eyeliner and mascara with 6% and 5%,respectively (Boxallet al.2008). The world-wide scale of use and production of C60 is unknown atthis point in time.Human health risk profileFullerenes exist in a variety of forms (carbon atom number, surface modifications, aggregationsstates, etc) and it is difficult to make generalisation about their toxic behaviour.So far it has been shown that one of the main factors for differences in fullerene toxicity seems tobe the water solubility with fullerenes of greater water solubility being less toxic.Fullerenes are assumed not to be effectively absorbed and to remain at the deposition site (mainlylung and gut). A small amount may be absorbed through the gut wall. The absorption via the skinis dependent on the fullerene type (functionallisation), the solvent/dispersant used and on skinproperties, but the dermal absorption is generally assumed to be low. Fullerenes seem to have avery low toxicity after oral exposure and an acute NOAEL of 2000 mg/kg bw/day.Following exposure via the pulmonary route fullerenes were able to induce pro- or anti-inflammatory responses with the factors driving these effects still unknown. An acute NOAEC of2.22 mg/m3 for acute inhalation is suggested as no inflammatory potential was seen at this con-centration.Identified studies suggest that fullerenes may not have an irritating potential to skin and eyes. Noconclusions can be made on the sensitising properties of fullerenes. Further clarification concern-
40
ing an irritating and sensitising potential of fullerenes is needed.No sub-chronic or chronic studies with fullerenes for any of the exposure routes have been identi-fied. The main effects associated with fullerene toxicity as shown byin vitroand acute toxicitystudies are inflammatory and oxidative responses as well as anti-inflammatory and anti-oxidantproperties.Identifiedin vitroandin vivomutagenicity /genotoxicity tests have reported contradicting results.Appropriatein vitro and in vivostudies should confirm the presence or absence of a mutageniceffect and the conditions influencing the test results.No information on carcinogenic effects of fullerenes has been identified and there are indicationsthat fullerenes may have anti-tumorigenic effects which could be an indication that carcinogenicityis not an endpoint of concern for fullerenes. However the identified information is not sufficient toreach this conclusion. The conclusion on whether carcinogenicity studies are needed is dependenton more information on toxicokinetic and mutagenicityEffects on female reproductive organs and embryos have been shown under quite artificial condi-tions. This endpoint should be re-evaluated once more information on the absorption and sub-chronic/chronic effects become available.Full physicochemical characterisation is essential to allow comparisons between fullerene toxicityand a risk assessment should focus on the fullerene type and concentrations to which humans areexposed.With regard to the human health risk there is little information available with regard to theamounts of nano C60used in different product types and thereby the extent of the exposure.Environmental risk profileIn general the acute ecotoxicity of C60seems to be low, however a LC50, 48h-value of 7.9 mg/1 hasbeen reported. This would rank C60as “Aquatic Chronic 2”. In longer-term exposure studies (32 d)with fish a NOEC of 0.04 mg/L and a LOEC of 0.2 mg/L for the length of juvenile carp has beenfound. Ecotoxicological testing of C60is difficult due to the very low water solubility of the com-pound and further method development may be needed. Tests performed using organic solventsdo not have a high credibility and should not be used for hazard identification or risk assessmentpurposes.Boxallet al.(2008) estimated regional concentrations of fullerenes in water to be in the order of0.3 �g/l. This estimate of the predicted environmental concentration (PEC) is highly uncertain dueto lack of data about the sources for fullerenes, amounts used, and the environmental transportand fate of fullerenes.As concluded by Stoneet al.(2010) the poor ecotoxicity database for fullerenes implies that noreliable estimation of predicted no-effect concentration (PNEC) can be made. Given the quality ofthe present PEC estimates and the lack of suitable ecotoxicity data for PNEC estimation, a quanti-tative risk characterization of fullerenes cannot be made at present.
41
42
4 Titanium dioxide - TiO24.1General characteristics
Titanium dioxide is the naturally occurring oxide of titanium. Often a distinc-tion is made by TiO2manufacturers between pigmentary grade and ultrafinegrade. The primary crystal size typically range from 150 to 300 nm for TiO22of a pigmentary grade and the surface area typically ranges from 6 to 60 m /g(coated and uncoated). The ultrafine grade typically has a primary crystal size2from 10 to 150 nm, and a surface area of between 50 to 200 m /g (coated anduncoated). The pigmentary TiO2has a white colour and is therefore widelyused in paints etc. The ultrafine TiO2including nano-sized TiO2is transpar-ent. A number of different crystal structures of TiO2exists of which the rutileand anatase forms of TiO2are the most important in relation to the use ofTiO2in consumer products. Nano-scale TiO2particles have various formsand shapes and may be cicular (non-spherical) or approaching sphericalforms. Nano-scale TiO2may furthermore display complex and various ag-glomeration structures. In contrast to the bulk TiO2(>100 nm) that is consid-ered chemically inert, nano-scale TiO2 can act as a photo-catalyst, that cangenerate reactive oxygen species upon illumination. Both UV-light and visiblelight can induce the catalytic activity of TiO2 and the anatase crystal form is amore efficient photocatalyst than the rutile form. The fotocatalytic effects canbe avoided by surface treatment e.g doping with other metals. Although nor-mally considered to be insoluble material, it can be made water dispersible byapplying certain surface treatments. In dry form, TiO2 nanomaterials willtend to agglomerate due to interaction of the particle surfaces, but the degreeof agglomeration is depending on the specific surface treatment, relative hu-midity, sample aging, etc. (ED & DuPont, 2007).4.2Manufacturing processes
A large number of manufacturing processes exist for the ultrafine grade, manyof which use either titanium tetrachloride or titanyl sulfate as starting material.These processes include precipitation, thermal hydrolysis and flame hydroly-sis. For the ultrafine grade, the crystal may be further processed involvingmilling, then coating and milling again. Depending on the medium relevantto the application for marketing, a possible last dispersion step (with water /cosmetic oils) can be applied, for example for UV attenuation dispersiongrades. If no further dispersion is carried out, the products obtained are UVattenuation powder grades. Both fine and ultrafine TiO2may be surfacetreated to increase their applicability in products, e.g. to ensure a uniform dis-tribution in sunscreens or to optimize UV-absorption properties.4.34.3.1UsesMain applications
TiO2is one of the most widely used chemicals in the world. As the bulk formof the substance often contains a nano-sized fraction, nano- TiO2has been
43
used for many years for different applications, without being identified asnano.A wide range of applications exists, exploiting the various properties of TiO2nanomaterials. The ability to scatter light can be manipulated by changing thesize of TiO2nanomaterials, hence pigmentary TiO2has been optimised forvisible light, which is applicable in white opaque paints, whereas ultrafineTiO2has been optimised for UV absorption and used in sunscreens. The abil-ity of TiO2to absorb UV radiation can be manipulated through surface treat-ment which is applicable to degradation of pollutants (NOx or dirt) or protec-tion of paint films and polymers from degradation from other chemical spe-cies (ED & DuPont, 2007). Thus, nano-scale TiO2is widely used in sun-screens and cosmetics due to the UV-absorption of the material.In paints and for water treatment, nano-scale TiO2is used as a photo-catalystproducing reactive oxygen that may degrade other organics. According to ED& DuPont (2007) the rutile form of nano-TiO2is most often used in applica-tions where low interaction with the surrounding matrix is desired, whereasuntreated anatase nano-TiO2is most often used in photocatalyst applications.A number of other and very diverse areas of application exists, such as oint-ments, toothpaste, catalysts, catalyst supports, adsorbents, delustrants, semi-conductors, etc. In total 21 products have been identified to be commerciallyavailable on the Danish marked through searches in the Woodrow Wilson In-stitute database. Most of these products fall into the category of 'Health andfitness'. In the category of 'Health and fitness' nano-TiO2is mostly used insunscreens (5), personal care products (4) and sporting goods (2), however itmust be stressed that these numbers are expected to underestimate the actualnumber of products in use in Denmark, since non-internet retailers are notincluded in the Woodrow Wilson Institute database.Thus, the number of sun-screen products and paints containing nano-TiO2is expected to be higherthan what the searches in the Woodrow Wilson Institute data base suggests.However, this can not be quantified, since the producers are not obliged todeclare whether or not their products contain nanoparticles.While TiO2rank as one of the most used chemicals world-wide (mainly as apigment), the tonnages of nano-scale TiO2used nationally, in the EU orworldwide can at present not be estimated. This is due to the fact that no spe-cific inventories for nanomaterials exist. However, it should be mentioned,that in some consumer products, e.g. sunscreens, the percentage of nano-TiO2may constitute several percent of the product (Barker & Branch, 2008;Hansenet al.,2008). TiO2(E171), which may contain nano-scale TiO2, isused for whitening and brightening food, especially for confectionary, whitesauces and dressings and certain powdered foods. TiO2is also used in thepharmaceutical industry as and opacity agent.It has been estimated, that in UK the dietary intake of TiO2(non-nano +nano) is 5 mg/person per day (Powell et al, 2010).Given the range of possibleapplications of nano-scale TiO2especially within photocatalysis (e.g. for self-cleaning surfaces and water treatment), the use of nanoTiO2is anticipated toincrease significantly in the near future.4.3.2Results from industry survey
The survey on TiO2has been focused on the use of TiO2as a pigment. TiO2is known to be widely used as a white pigment in paint, plastics, mortar, ce-
44
ment, toothpaste, cosmetics and in most other cases, where a white pigment isneeded. TiO2is however also used in combination with other pigments to ob-tain different shades of e.g. grey, green etc.The concentration of TiO2differs depending on the material in question andthe colour strength required. In paint the concentration of pigments will typi-cally be in the range of 0.1-10% (Environmental project No. 1206, 2007).The consumption of TiO2in Denmark may be roughly estimated to be above1000 tons/year. An import of pigments and preparations containing TiO2of2about 1800 tonnes to Denmark was registered for 2009 .4.4Eco-toxicological profile
While bulk-scale TiO2is generally believed to be inert and not expected to behardous to the environment, nano-scale TiO2concerns have been raised, sincenovel properties at the nano-scale, e.g. photo-catalytic activity, may changethe hazard profile of TiO2.The ecotoxicological profile of nano-scale titaniumdioxide (TiO2NP) is buildupon original research papers, but it should be stressed that only a fewecotoxicological studies exist and that none of these have been aimed at verifi-cation/reproducing the effects found. Thus, it is still unknown whether thepatterns observed for the ecotoxicity of TiO2NP later on will be proven to besingle events.For fish there are strong indications that even though the acute toxicity ofTiO2nanoparticles is low, effects are observed in the more detailed studiesfocussed at gills, brain, and intestine (Federiciet al.,2007; Ramsdenet al.,2009). This implies that other endpoints than the traditionally used ones maybe relevant for nanoparticles.For crustaceans, TiO2NP has been found to show both acute and long-termtoxicity as indicated by the values reported by Zhuet al.(2010): LC50, 72hof 2.02 mg/L and EC50, 21d (survival of offspring) of 0.46 mg/L. This wouldcorrespond to a classification in the hazard class “hazardous to the aquatic3environment” according to the CLP . However, it should be emphasized thatthe results of Zhuet al.(2010) are in contrast to other studies that have foundLC50 values of around 2000 mg/L (Heinlaanet al.,2008) and >10 mg/L(Griffittet al.,2008) and a 40% mortality at the 20 mg/L (Adamset al.,2006). In a 21-day study using Daphnia magna and 40 nm TiO2NP, Kimetal.(2010) found a significantly increased mortality at concentrations of 5 and10 mg/L, but no reduction of the reproduction ability was observed. Kimet al.(2010) suggest that oxidative stress in D. magna due to reactive oxygen spe-cies may explain the TiO2NP toxicity. At present the large spread in the re-ported data makes it impossible to make a definitive conclusion on the toxicityof TiO2NP on crustaceans.For algae, the lowest EC50, 72h for TiO2nanoparticles is 5.83 mg/L (Aroujaet al.,2008) and this study also found that TiO2NP were more toxic than theDanmarks Statistik, 2010REGULATION (EC) No 1272/2008 OF THE EUROPEAN PARLIAMENTAND OF THE COUNCIL of 16 December 2008 on classification, labelling andpackaging of substances and mixtures, amending and repealing Directives67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/200632
45
corresponding bulk particles (EC50, 72h estimated to 35.9 mg/L). NOEC ofthe bulk materials was found to be 10.1 mg/L for bulk TiO2as compared to0.98 mg/L for TiO2NP (Aruojaet al.2008). However, the low effect valuesfound by Aroujaet al.(2008) and Wanget al.(2008) may not be reliable,considering the experimental challenges in the algal test as described byHartmannet al.(2010). It seems unlikely that Aroujaet al.(2008) did not en-counter the same kind of problems with reproducibility and agglomerationduring testing using uncoated TiO2particles. Therefore, it is recommendedthat the lowest of the values found by Hartmannet al.(2010) is used in haz-ard assessment of TiO2NP, i.e. the EC50, 72h of 71.1 mg/L.So far only three studies of bioaccumulation of TiO2has been published. Zhuet al.(2010) found that 21 nm TiO2nanoparticles were accumulating in D.magna and that it was increasing exposure time. Due to a relatively slowelimination of TiO2 from D. magna the authors report very high BCF values(>10,000) based on concentrations in daphnids versus water phase concen-trations (Zhuet al.,2010). However, by calculating BCF values from the up-take and depuration rates, BCF values <100 can be estimated. In fish, Ram-denet al.(2010) found Ti-accumulation in the gill, gut, liver, brain and spleenof rainbow trout (Oncorhynchus mykiss) after 8 weeks of dietary exposure to10 or 100 mg/kg bw TiO2nanoparticles. In some of these organs, especiallythe brain, no clearance of Ti occurred after exposure. No estimations of BCFwere made in this study. After intravenous injection of TiO2nanoparticles inRainbow trout (Oncorhynchus mykiss), Scownet al.(2010) found increasedlevels of Ti in the kidneys and the Ti remained there for up to 21 days. Evi-dence of clearance was, however, noted after 90 days. Ti accumulation in theliver was 15 times lower than in the kidney, but no apparent clearance tookplace in the liver.By definition metal oxide nanoparticles are not degradable and speciation ofTiO2(e.g. dissociation or complexation) is not likely to occur in aquatic mediaunder normal conditions. Therefore, TiO2particles (both bulk and NP) canbe regarded as persistent in the sense that the particles will remain TiO2-particles, however a number of factors may influence the particle size and alsothe resulting bioavailability in aquatic media (Hartmannet al.,2010).4.5Toxicological profile
Due to its widespread exploitation, a great number of studies have focussedon revealing the toxicity of titanium dioxide (TiO2). Such studies have ad-dressed the consequences of exposure via a number of routes. TiO2(bulkform) exposure via the pulmonary route has included the use as a control par-ticulate compared to pathogenic materials such as alpha-quartz, but has alsobeen studied in relation to occupational exposures. Dermal penetration andtoxicity of TiO2particles have also formed the focus of a number of studiesdue to their inclusion within sunscreens and cosmetic products. In a few stud-ies toxicity was investigated after intraperitoneal and oral exposure. Humansare exposed orally to high concentrations of TiO2 particles via food. Thetoxicological evaluation of TiO2is mainly quoted from ENHRES (Stone e.al,2010). In addition the following reviews were used; IARC, NIOSH (2005)and Jelnes et al (1997).As mentioned earlier, bulk TiO2is considered chemically inert and of low tox-icity. Adverse lung effects associated with TiO2are primarily a result of a gen-eral particle overload more than a specific toxic effect of TiO2. In relation to
46
nano-scale TiO2it is therefore of particular interest to evaluate if the nano-forms have specific toxic properties.4.5.1ADME studies
It is necessary to consider the potential for particle absorption from the site ofexposure into the blood, as this will dictate their systemic availability and dis-tribution within the body.Absorption and distributionNo studies were identified investigating systemic absorption after exposure viainhalationof TiO2nanoparticles. Many studies were performed on toxicityafter inhalation, but the issue on systemic absorption has not been reported.There is evidence of pulmonary retention of TiO2nanoparticles after expo-sure via inhalation (Ferin et al., 1992 and Bermudezet al.,2004). Wanget al.(2008a and 2008b) illustrated the transfer of TiO2nanoparticles to the brain,followingintranasal exposure,which occurred via their transport within neu-rones.TiO2nanoparticles were observed to translocate into the blood, followingoralexposure,and thereafter distribute to secondary targets, including the liver,spleen, lungs, and kidneys (Wanget al.,2007, 2008). However it is not pos-sible to evaluate the degree of absorption based on the data available.Studies investigatingdermal exposureof TiO2in vivo(humans) andin vitro(porcine and human skin) suggest that penetration of nanoparticles past thestratum corneum is negligible, and therefore it is unlikely that the particles willaccess the circulation via this route for intact skin, whereas more knowledgehas to be gained on damaged skin (Stoneet al.,2010).Metabolism and eliminationNo evidence of TiO2 metabolism, or elimination from the body could beidentified in the literature at this time.4.5.2Short term toxicity
Acute inhalation toxicityIn several studies there is clear evidence that nano-sized TiO2is considerablymore toxic than microsized TiO2( Ferinet al.,1992; Renwicket al.,2004;Chenet al.2006; Inooueet al.2008). In addition, it was found that the crys-tallinity (or the specific crystal form) of TiO2nanoparticles is thought to influ-ence the toxicity, with the anatase form expected to be more toxic than therutile form (Warheitet al.,2007). The pulmonary response to TiO2 is in-flammation (Ferinet al.,1992; Chenet al.,2006; Warheitet al.,2005; War-heitet al.,2007; Inoueet al.,2008; Renwicket al.,2004; Grassianet al.,2007), epithelial damage, increased permeability of the lung epithelium, oxi-dative stress and cytotoxicity (Renwicket al.,2004), and morphological al-teration within the lung (Chenet al.,2006). In the Renwicket al.study (2004)no toxicity was observed at 125�gper rat corresponding to 0.5�g/kgbw, as-suming a rat body weight of 250 g) whereas toxicity (inflammation and histo-logical changes in the lung) was seen at 500�gper rat (pareticle size 29 nm).A NOAEL for toxicity of 0.5�g/kgbw was proposed by Stoneet al.,2010.
47
Chenet al.(2006) exposed mice, via intratracheal instillation (0.1 and 0.5 mgper mouse), to nano (19-21 nm) and micro (180-250 nm) forms of TiO2,anddetermined their pulmonary toxicity 3 days, 1 week or 2 weeks post exposure.In mice exposed to TiO2,particles (size 19-21 nm) toxicity (inflammation andhistological changes in the lung) was observed at the lowest dose of 100μgper mouse (Chenet al.,2006). Despite the huge doses used, no pathologicallesions were found in response to microparticulate TiO2.Acute dermal toxicityThe impact of TiO2,exposure on the skin is especially relevant due to the in-clusion of such particles within sunscreens and cosmetics that are directly ap-plied to skin.In relation to acute dermal toxicity, noin vivostudies have been identified.However, Kisset al.(2008) and Jinet al.(2008) (as cited by Stoneet al.,2010) have shown that TiO2nanoparticles may affect different cell linesinvitro.The findings from the available studies demonstrated that the penetration ofmicrosized-TiO2is negligible within the “healthy” skin. However, sunscreensare often applied to burnt, damaged and diseased skin, where the barrier func-tion of the stratum corneum will inevitably be impaired (Bormet al.,2006;Kisset al.,2008) (as cited by Stoneet al.,2010), which should be taken intoconsideration in future studies.Acute oral oxicityAlthough high human exposure via the oral route has been estimated (Powellet al.,2010) only one study could be identified that investigated acute oral ex-posure to TiO2. Wanget al.(2007) investigated the distribution and acutetoxicity of nanoparticulate (25 and 80 nm) and microparticulate (155 nm)forms of TiO2,following the oral exposure (5 g/kg bw) in mice. TiO2,particlesof all sizes were distributed to the liver, spleen, lungs, kidneys, thus providingevidence that they could be transported to other sites subsequent to exposure,due to their translocation into the blood. The primary target organ fornanoparticles was the liver (an inflammatory response was detected). Markersof cardiac damage were also observed to be elevated by TiO2nanoparticles.Microparticulate TiO2induced limited toxicity.Acute intraperitoneally toxicityThe only study that used intraperitoneal injection (Chenet al.,2008) investi-gated the acute toxicity of TiO2,nanoparticles (80-100 nm) subsequent to in-traperitoneal injection, of mice. The doses used were exceptionally high(ranging from 324 to 2592 mg/kg), and it is therefore not surprising that mor-tality was associated with exposure. Furthermore, TiO2was observed to blockpulmonary vessels, leading to thrombosis, with pathology also evident withinthe liver, spleen and kidneys. This study highlights the need to consider theuse of relevant doses of particles, as the observed effects derived from the highdose and not the inherent toxicity of particles.4.5.3Irritation and corrosion
No studies specifically investigating irritation (dermal, eye and inhalation)have been identified. However, as TiO2nanoparticles are used widely in sun
48
creams, they are not expected to be irritating to the skin. It is also not ex-pected that these nanoparticles are irritating following inhalation.4.5.4Skin and respiratory sensitization (in vitro
andin vivo
)
No studies specifically investigating sensitization (dermal, eye and inhalation)have been identified. However, as TiO2nanoparticles are used widely in suncreams, they are not expected to be sensitizing to the skin. It is also not ex-pected that these nanoparticles are sensitizing followig inhalation.4.5.5Repeated dose toxicity, short term, sub-chronic and long term
Repeated dose: inhalationThe greatest number of studies on TiO2, by far address the consequences ofthe exposure of the lungs, and in particular the size dependence of any effects.The studies performed on pulmonary exposure of TiO2shows that the toxic-ity primarily was dictated by particle size, and crystal structure; whereby de-creasing particle size, and anatase forms of TiO2enhanced particle toxicity.In a 12 week inhalation study Ferinet al.(1992) (as cited by Stone et al,2010) showed that TiO2nanoparticles caused a pulmonary inflammation inrats, which micro-TiO2(250 nm) did not at the same dose levels (thus con-firming the potency of TiO2nanoparticles as discussed within the short termtoxicity section).In addition, it is suggested that the exposure scenario (including species used,exposure method and dose administered) can impact on the toxicity of TiO2and other metal oxides. It has been shown in a 13 weeks (6 hours per day, 5days per week, 21 nm particles) inhalation study that rats are more sensitivethan mice and much more sensitive than hamsters Bermudezet al.(2004). Asthe rat is the most sensitive species it was used for risk assessment in the EN-RESH report. From this study a No Observed Effect Concentration33(NOAEC) of 0.5 mg/m (500μg/m) was identified.It has been shown in a 30 days study that both rutile and anatase TiO2nanoparticles after nasal installation of 500�g/mouseevery other day for atotal of 30 days can bypass the blood brain barrier and be translocated (via theolfactory nerve) to the brain, where they accumulated within the cerebral cor-tex, thalamus and hippocampus (main target). This resulted in morphologicalalterations and loss of neurones in the hippocampus, induction of oxidativestress and initiation of inflammation (Wanget al.,2008a, 2008b)After chronic exposure (2 years) of rats to TiO2nanoparticles, at an average3exposure concentration of 10 mg/ m , lung tumours were observed. However,the relevance of these data for risk assessment purposes are dubious due to the3very high doses used (average exposure concentration of 10 mg/m ), and thehigh mortality: 60% after 24 months (control 40%) and 90% after 130 weeks(control 85%) (Heinrichet al.,1995).Repeated dermal doseNo studies related to repeated dose toxicity via the dermal route were avail-able
49
Repeated oral doseNo studies related to repeated dose toxicity via the oral route were available4.5.6Mutagenicity/genotoxicity
There is little information available concerningin vivogenotoxicity of nanoTiO2. Driscollet al.(1997) investigated the mutagenic effect at the hprt locusin rat alveolar epithelial cells after intratracheal installation of anathase TiO2(median diameter = 180 nm). TiO2induced mutations at 100 mg/kg bw butnot at 10 mg/kg bw. The potency of TiO2was compared withα-quartspar-ticles (mean diameter = 900 nm) and carbon (mean diameter = 15 nm).Α-quarts was the most potent followed by cabon black and TiO2being the lesspotent. All particles induced a neutrophilic inflammatory respond which cor-related with the relative genotoxic potency of the different materials. Thesefindings indicate that inflammation may play a key role of thein vivogenotoxic effect of insoluble particles, supporting a non-linear (thresholded)relationship between particle exposure and mutagenicity.In a recent paper Trouilleret al.,(2009) investigated the genotoxicity, oxida-tive DNA damage and inflammation of nano-TiO2in anin vivostudy in mice.The crystal structure was a mixture of 75% anatase and 25% rutile TiO2witha particle size of 21 nm. Male mice were dosed for 5 days in the drinking wa-ter with doses corresponding to 0, 50, 100 250 and 500 mg/kg bw. Pregnantdams were dosed in drinking water with 500 mg/kg bw for 10 days at gesta-tion days 8.5 to 18.5 post-coitum. In male mice nano-TiO2induced DNAsingle strand breaks measured by the comet assay at the highest dose tested(500 mg/kg/bw) and micronuclei in peripheral blood. DNA double strandbreaks (DBS) was measures byλ-H2AXimmunostaining assay in bone mar-row, which was the most sensitive of the assays involved, and showed an in-crease in DBS in a dose dependent manner. Oxidative DNA damage (8-OHdG) was measured in the liver at the highest dose tested, and a proin-flammatory response, measured as changes in cytokine expression, was seenin peripheral blood. In utero exposure of fetuses via the mother caused an in-crease in large deletions in offspring.Thisin vivostudy show that the investigated nano-TiO2administrated orallyis systemically distributed to different tissues such as blood, bone marrow,liver and even the embryo, where it can induce genotoxicity at relatively highexposure levels. The inflammatory response and oxidative damage in liverindicate that the mechanism behind the observed genotoxicty may be due to asecondary response following inflammation and oxidative stress.Although the genotoxic effect observed after oral exposure might be thresh-olded, concern is raised due to its carcinogenic potential following chronicexposure (Heinrichet al.,1995), and because of the potential to induce ge-netic disorders in the offspring as indicated by Trouilleret al.(2009).Noin vitrogenotoxicity/mutagenicity studies were identified using the classi-cal tests used in regulatory settings. However, some evidence of genotoxicityhas been encountered within a number ofin vitrostudies.Rahmanet al.(2002) (as cited by Stoneet al.,2010) investigated the potentialfor TiO2to elicit chromosomal aberrations in SHE fibroblasts. Micronucleiwere evident within cells exposed to nano (<20 nm), but not micro (>2002nm) TiO2particles (up to 10 �g/cm , for up to 72 hours). The nanoparticles
50
also triggered the induction of apoptosis within cells, which is recognised as acommon response to DNA damage.It was shown by Karlssonet al.(2008) (as cited by Stoneet al.,2010) thatTiO2nanoparticles were able to elicit DNA damage, as determined by theComet assay in A549 lung epithelial cells.Nakagawaet al.(1997) investigated the genotoxicity of TiO2nanoparticles (inrutile and anatase forms), in the absence or presence of UV light. WithoutUV light, TiO2nanoparticles induced no, or very limited genotoxicity. How-ever in the presence of UV light TiO2elicited DNA damage and chromosomeaberrations (but no gene mutations). The type of cells used for the test is notmentioned. The genotoxic effect of TiO2seems to be influenced by the chrys-taline form with the anatase form being more potent that the rutile form.The UV dependence of the genotoxic potential of TiO2nanoparticles (inrutile and anatase forms) was also investigated by Theogarajet al.(2007). Incontrast to the previously discussed investigations, no chromosomal altera-tions were observed within CHO cells, either in the presence or absence ofUV light.The genotoxicty of TiO2nanoparticles is thought to be driven by particle me-diated ROS production. The particles themselves are not thought to be inher-ently genotoxic, but may trigger genotoxicity via an indirect threshold driveninflammatory mechanism involving oxidative stress and in particular neutro-phil presence (Driscollet al.,1997).4.5.7Carcinogenicity
Only one study on carcinogenicity was identified Heinrichet al.(1995) and isdescribed under repeated dose toxicity above. In this chronic inhalation studyvery high doses were used, and the study had a long duration, resulting inhigh death, also in the control group.Based on this data, NIOSH (2005) concluded, that TiO2is carcinogenic inrats and that it cannot be excluded as carcinogenic in humans. It is expectedthat carcinogenicity occurs following pulmonary particle overload and thushas a threshold and that the effects are considered to be caused by the particleexposure rather than the specific chemical substance. The InternationalAgency for Research on Cancer have assessed TiO2(even the microform – ifexposure is high enough) to be a Class 2B carcinogen (Possibly carcinogenicto humans) (IARC 2006).4.5.8Reproductive toxicity, developmental toxicity and teratogenicity
Evaluation of metal oxide nanoparticle effects on the reproductive system islimited to a small number ofin vitrostudies.Komatsuet al.(2008) determined the potential for TiO2nanoparticles, dieselexhaust particles and ultrafine carbon black to impair the male mouse repro-ductive system. This study evaluated the direct effect of nanoparticles on tes-tis-constituent cells, and examined the effect of TiO2on mouse Leydig TM3cells, the testosterone-producing cells of the testis. TiO2was more cytotoxic toLeydig cells than the other carbon based particles used in the study. The pro-liferation of Leydig cells was suppressed transiently by treatment with TiO2.
51
TiO2nanoparticles were taken up by Leydig cells, and in turn affected cellviability, proliferation and gene expression.No literature examining nanoparticle effects on organs or cell types in the fe-male reproductive system were found.4.5.9Summary
The bulk (microsized) form of TiO2is generally regarded as reletively inert,with a low toxic potential (Jelnes, 1997).However, in several studies there is clear evidence that nano-sized TiO2ismore toxic than micro-sized TiO2. First of all nano-sized TiO2is absorbedfrom the gastrointestinal tract and distributed to secondary target organs,whereas microsized TiO2is not absorbed via this exposure route (Jelnes,1997). It has been consistent within a wide range of investigations that theacute toxicity increases as particle size decreases. In addition the toxicity isdependent on the structure of the particles, with the anatase form being mosttoxic. The toxicity of TiO2nano-particles has been demonstrated to be in-flammation, induction of oxidative stress, and histological changes in the tar-get organs. The primary target organ after inhalation is the lung. It is assumed3(Stoneet al.,2010) that the NOAC is somewhere between 0.5 to 2 mg/m . Afew studies indicate the liver is the primary target organ after oral exposure,and that TiO2exposure also may be related to cardiac damage.There is indication fromin vitrostudies that nano-sized TiO2but not micro-sized TiO2is genotoxic, and that the genotoxic response is caused by oxida-tive stress, assuming a thresholded mechanism.The genotoxic effect ofin vitroof TiO2has been confirmed in twoin vivostudies, which also indicate that genotoxicity is due to inflammation andoxiditative stress.Although the genotoxic effect observed after oral exposure might be thresh-olded, concern is raised due to its carcinogenic potential following chronicexposure (Heinrichet al.,1995), and because of the potential to induce ge-netic disorders in the offspring as indicated by Trouilleret al.(2009).The inflammogenic, oxidative, and indirect genotoxic effects of nano-sizedTiO2are considered to be inherently linked.Future needs for researchAlthough nano-sized TiO2is one of the most well investigated nano sub-stances, several data gaps still exist in relation to toxicological evaluation.None of the studies performed and reviewed in this survey is in accordancewith the requirements in international accepted guidelines for toxicity testingaccepted for regulatory purposes. The responses observed within a few stud-ies were measured following relatively high exposure doses. In order to clarifywhether the observed effects, e.g. carcinogenicity is of biological relevance,further studies following international accepted guidelines are needed. Thisespecially important because nano-sized TiO2has shown genotoxic potentialin vivoin different organs and even in the fetus after exposure of the mother.Therefore relevant reproductive studies are also needed.
52
For the TiO2samples, variations in crystalline structure suggest that it mightbe difficult to compare across studies and to generate an overall conclusion.Clearer characterisation of the particles used is required within studies in or-der to identify the attributes of TiO2that are most influential in driving toxic-ity.It can also be suggested that systemic responses, following pulmonary expo-sure are possible. However, this would require further investigations, whichcould also focus on determining the factors responsible for such a response, ifthe particles cannot directly access the circulation. In addition, the neuronaltransport of nanoparticles has been demonstrated, which offers a potentialroute for nanoparticle distribution within the body, and enables them to by-pass the blood brain barrier, and thereby access the brain. Further studies forexamining the consequences of this exposure route are needed to put thesepotential CNS effects in a risk assessment perspective.Studies have mainly focused on dermal and pulmonary toxicity of particles,but there is an absence of data on the consequences of exposure to dam-aged/diseased skin, and few data are available on toxicity after oral exposure.Thus further data is needed. Other relevant target organs include the liver,kidney, cardiovascular system and brain which is necessary due to the factthat nanoparticles are likely to become systemically or neuronally available.The liver could be highlighted as a priority due to the propensity of particlesto accumulate in this organ.4.6Exposure scenarios
For consumers the highest direct exposure to nano-scale TiO2is expected tobe through the use of sunscreens and dermal contact is the most likely route ofexposure unless the products are applied as sprays. For sunscreens containing2% nano-scale TiO2, Hansenet al.(2008) estimated a worst-case exposure of57 �g/kg bw/d for a 2-year old child.TiO2is also used in paints, varnishes and inks and coatings/surfaces to whichconsumers can be exposed either via the dermal or inhalation route (sprays)The only consumer exposure data available, reported very low exposure (223particles/m , 55 nm) for release from coatings due to wear and tear, which willprobably not constitute a risk.TiO2is used in spray applications, but no exposure data for consumers areavailable. Based on modelling of application of a spray-on sunscreen, a maxi-3mum value of 3.5 g/m for short term exposure is estimated.In addition humans are also exposed to high amounts of TiO2via food, andan unknown part of this will be on nanoform. It has been estimated that inUK the dietary intake of TiO2is 5 mg/person pr day. (Powellet al.,2010).This may raise concern because nano-sized TiO2, has a genotoxic potentialafter oral exposure, but presumably by a non-linear (thresholded) mechanism.The widespread uses of TiO2in consumer products like sunscreens, surfacetreatment and paints allow for emissions of TiO2nanoparticles to the envi-ronment during and after application e.g. via wastewater or leaching ofnanoparticles from treated surfaces.
53
In the study by Kaegiet al.(2008) it was found that TiO2nanoparticlesleached from painted house facades resulting in a concentration of 8 �g/l inurban runoff. Using modelling approaches, Müller and Nowack (2008) calcu-lated PEC-values between 0.7 and 16 �g/l in water and Boxallet al.(2008)estimated environmental concentrations of 24.5 �g/l TiO2nanoparticles in wa-ter. Both of these modelling attempts are based on assumptions that cannot bevalidated, however it should be noted that they give results in the same orderof magnitude.4.74.7.1Risk profileEnvironment
The ecotoxicity of bulk TiO2(particles >100 nm, typically with a size around300 nm) is at present very poorly documented. Thus, reliable comparisonsbetween nano-sized and bulk TiO2are difficult at present. Only a few of theecotoxicity studies have reported the results in terms of the endpoints andvalues listed in Information Requirements of the REACH (ECHA, 2008).The lowest reported EC50 and LC50 values are in the range 1-10 mg/L (al-gae and crustaceans). Detailed studies on fish indicate that other endpointsthan the traditionally used ones may be relevant and focus should be directedtowards gills, brain, and intestines.Bioaccumulation of TiO2NP has been shown in aquatic species however moreresearch is needed to quantify this in terms of reliable BCF values. TiO2parti-cles (both bulk and NP) can be regarded as persistent in the sense that theparticles will remain TiO2-particles upon release to the environment.TiO2nanoparticles have been found to be released to the environment fromthe different uses, e.g. outdoor paints and personal care products. Upon con-tact with water, uncoated TiO2nanoparticles tend to aggregate and settle,however the presence of natural organic matter may have a stabilizing effect.In Stoneet al.(2010) a PNEC of 5.8 �g/l for freshwater has been estimatedusing an assessment factor of 1000. Based on comparisons with modelledPEC-values, the risk quotient for TiO2nanoparticles (PEC/PNEC) assumesvalues from 0.1-4.2, indicating that TiO2may give rise to toxicity in theaquatic environment. However, very large uncertainties exist not only withrespect to obtaining valid ecotoxicological results for TiO2nanoparticles(Hartmannet al.,2010), but also for estimating environmental concentrationsand for taking bioavailability into account when estimating e.g. PNEC. Thelack of information on production volumes, amounts of TiO2nanoparticles inproducts, and market penetration makes all estimates of PEC very uncertain.4.7.2Human health
TiO2nanoparticles were shown to be absorbed following oral exposure, andthe main target organ seems to be the liver. There is however too few data toperform a risk characterisation for oral exposure.There is substantial evidence that nano-TiO2does not pass through healthyskin, and it can be concluded that healthy skin is most likely not a risk, but itmight be a risk for damaged skin.
54
No DNEL can be derived for short term and chronic oral and dermal expo-sure.There is substantial evidence that following inhalation nano-TiO2is moretoxic than microsized TiO2, and the effect is dose dependant. There seems todifference in sensitivity between species, with the rat being most sensitive.The the crystalline form seems also to influence toxicity, with the anataseform being more toxic than the rutile form.Based on a NOAC of 0.5 mg/m3 from a 13 week repeated dose toxicity study3(Bermudez et al, 2004) a DNEL = 17�g/mfor chronic inhalation (workers 8h a day, 21 nm particles) was calculated based on corrections factors in theREACH guidance (ECHA 2008).There is no data available to derive a DNEL for acute inhalation exposure.In general some data are available for dermal exposure but no indication ofdirect adverse effects are observed. More information is needed to completethe picture regarding absorption and to allow robust risk assessment. How-ever, there are indications that the risk from exposure to healthy skin is lowdue to a probable negligible uptake. Fototoxicity cannot be excluded for cer-tain TiO2qualities. Documentation is avalible with regard to toxicity and ad-verse effects as a result of inhalation but in general TiO2is likely to be associ-ated with low risk from exposure via consumer products unless applied as air-borne particles.Few toxicity data are available after oral exposure. Although it is assumed thatthe genotoxic effect observed inin vivois thresholded, a NOAEL has notbeen derived. Long term oral toxicity studies are needed in order to perform arisk assessment.4.8Summary sheet for TiO2
Nanomaterial characteristicsNameCAS numberChemical compositionAppearanceManufacturingessesproc-Titanium dioxide13463-47-7TiO2White powderSource: wikipedia
Manufacturing processes include precipitation, thermal hydrolysis andflame hydrolysis using titanium tetrachloride or titanylsulfate as startingmaterial. For the ultrafine grade, the crystal may be further processedinvolving milling, then coating and milling again.
Nanomaterial descriptionTitanium dioxide is the naturally occurring oxide of titanium. Often a distinction is made by TiO2manufacturers between pigmentary grade and ultrafine grade. The primary crystal size typicallyranges from 150 to 300 nm for TiO2of a pigmentary grade. The ultrafine grade typically has a pri-mary crystal size from 10 to 150 nm. The pigmentary TiO2has a white colour and is thereforewidely used in paints etc. The ultrafine TiO2including nano-sized TiO2is transparent. The rutileand anatase crystal forms of TiO2are the most important in relation to the use of TiO2in con-
55
sumer products.In contrast to the bulk TiO2(>100 nm) that is considered chemically inert, nano-scale TiO2and inparticular the anatase form can act as a photo-catalyst that can generate reactive oxygen speciesupon illumination. Both UV-light and visible light can induce the catalytic activity of TiO2and theanatase crystal form is a more efficient photocatalyst than the rutile form. Although normally con-sidered to be insoluble material can be made water dispersible by applying certain surface treat-ments. In dry form, TiO2nanomaterials will tend to agglomerate due to interaction of the particlesurfaces, but the degree of agglomeration is depended on the specific surface treatment, relativehumidity, sample aging, etc. Both fine and ultrafine TiO2may be surface treated to increase theirapplicability in products, e.g. to ensure a uniform distribution in sunscreens or to optimize UV-absorption properties.ApplicationsA wide range of applications exists for TiO2nanomaterials exploiting the various properties of TiO2nanomaterials. Pigmentary TiO2is widely used as a pigment in paints, whereas nano-scale TiO2iswidely used in sunscreens and cosmetics due to the UV-absorption of the material. In paints andfor water treatment nano-scale TiO2is used as a photo-catalyst producing reactive oxygen that maydegrade organic contaminants. Finally, a number of other and very diverse set of applications ex-ists such as ointments, toothpaste, catalysts, catalyst supports, adsorbents, delustrants, semi-conductors, etc. In some consumer products, e.g. sunscreens, the percentage of nano TiO2mayconstitute several percent of the product. TiO2rank as one of the most used chemicals world-wide(mainly as a pigment), but the tonnages of nano-scale TiO2used nationally, in the EU or world-wide can at present not be estimated. Given the range of possible applications of nano-scale TiO2,the use is anticipated to increase significantly in the near future.Human health risk profileThe bulk (microsized) form of TiO2is generally regarded as reletively inert, with a low toxic poten-tial. However, in several studies there is clear evidence that nano-sized TiO2is more toxic thanmicro-sized TiO2. First of all nano-sized TiO2is absorbed from the gastrointestinal tract and dis-tributed to secondary target organs, whereas microsized TiO2is not absorbed via this exposureroute It has been consistent within a wide range of investigations, that the acute toxicity increasesas particle size decreases. In addition the toxicity is dependent on the structure of the particles,with the anatase form being most toxic. The toxicity of TiO2nano-particles has been demonstratedto be inflammation, induction of oxidative stress, and histological changes in the target organs.The primary target organ after inhalation is the lung. It is assumed (Stone et 2010) that the NOACis somewhere between 0.5 to 2 mg/m3. A few studies indicate the liver is the primary target organafter oral exposure, and that TiO2exposure also may be related to cardiac damage.There is indication fromin vitroandin vivostudies that nano-sized TiO2but not micro-sized TiO2is genotoxic, and that the genotoxic response is caused by oxidative stress, assuming a thresh-olded mechanism.The inflammogenic, oxidative, and indirect genotoxic effects of nano-sized TiO2are considered tobe inherently linked.Environmental risk profileThe bulk (microsized) form of TiO2is generally regarded as relatively inert, with a low environ-mental risk. For nano TiO2the lowest reported EC50 and LC50 values are in the range 1-10 mg/L(algae and crustaceans). Detailed studies on fish indicate that other endpoints than the tradition-ally used ones may be relevant and focus should be directed towards gills, brain, and intestines.Bioaccumulation of TiO2NP has been shown in aquatic species however more research is neededto quantify this in terms of reliable BCF values. TiO2particles (both bulk and NP) can be regardedas persistent in the sense that the particles will remain TiO2-particles upon release to the environ-ment.TiO2nanoparticles have been found to be released to the environment from the different uses, e.g.outdoor paints and personal care products. Upon contact with water, uncoated TiO2nanoparticlestend to aggregate and settle, however the presence of natural organic matter may have a stabiliz-ing effect.In Stoneet al.(2010) a PNEC of 5.8 �g/l for freshwater has been estimated using an assessmentfactor of 1000. Based on comparisons with modelled PEC-values, the risk quotient for TiO2nanoparticles (PEC/PNEC) assumes values from 0.1-4.2, indicating that TiO2may give rise to risk
56
in the aquatic environment. However, very large uncertainties exist not only with respect to obtain-ing valid ecotoxicological results for TiO2nanoparticles, but also for estimating environmentalconcentrations. The lack of information on production volumes, amounts of TiO2nanoparticles inproducts, and market penetration makes all estimates of PEC very uncertain.
57
58
55.1
Zero valent iron - nZVIGeneral characteristics
Nano-scale zero-valent iron is the nanoform of zero valent iron. In its most0basic form it consists of spherical iron (Fe ) nanoparticles with individual par-ticle dimensions less than 100 nm. Substantial variations exist in the proper-ties of nZVI in regard to average particle size, particle size distribution, spe-cific surface area, surface charge and the presence of trace metals. nZVI mayfurthermore often be coated in order to prevent agglomeration and better con-trol their reactivity and mobility. Polymers, polyelectrolytes, and surfactantsare among the main types of coatings used for nZVI, including starch,poly(acrylic acid) (PAA) , poly(styrene sulfonate), etc. The lifetime of thesecoatings is generally unknown (Griegeret al.2010).5.2Manufacturing processes
A number of different synthesis methods exist including the Sol-gel methodsor chemical solution deposition methods where the solution acts as the pre-cursor for an integrated network or gel as described by Changet al.(2005),and the method of ferric iron reduction with sodium borohydride as describedby Sunet al.(2006) and Liet al.(2006) (Griegeret al.2010).5.35.3.1UsesMain applications
Not many applications of nZVI are known to us at this point and no con-sumer products have been identified to be commercialized in the Danish mar-ket. In the nanoform, ZVI have significantly increased available reactive sur-face areas which have been found to subsequently enhance contaminant deg-radation reactions. This has been studied in pilot studies inin situsoil reme-diation at contaminated sites (PCBs, chlorinated organic solvents, and or-ganochlorine pesticides) not easily accessed by other treatment methods(Griegeret al.2010). To the best of our knowledge no field scale applicationsof nZVI has been undertaken in Denmark. Some medical applications havealso been reported, but it is the use in soil and groundwater remediation that isanticipated to be the major use of nZVI in the future.5.3.2Results from industry survey
No further information about nZVI has been available.5.4Eco-toxicological profile
For the following overview of the ecotoxicological profile of nano-scale zero-valent iron it should be stressed that only a few ecotoxicological studies existand only one of these is aimed at the base-set organisms (fish, crustacean andalgae) required for doing effects assessment according to REACH.
59
In a study with bacteria (E. coli), it was found that nZVI is bactericidal underanoxic conditions and that the toxicity was significantly lower under oxygensaturated conditions (Leeet al.,2008). It was suggested that Fe(II) releasedfrom nZVI contributes to toxicity and that the toxic mechanism is oxidativestress induced by reactive oxygen. nZVI was found to cause a significantphysical disruption of cell membranes.Based on extensive literature searches in ISI Web of Science it is found thatno studies have been published in peer reviewed journals (until 22 October2010) on the ecotoxicity of nZVI to algae and crustaceans. Only one peer-reviewed study (Liet al.,2009) has been carried out test with fish (Medaka,Oryzias latipes). It was found that nZVI caused disturbance in the oxidativedefense system for embryos and adults, as well as oxidative damage in em-bryos. Observed effects occurred at concentrations down to 0.5 mg/L. Inadult fish a disturbance of the antioxidant balance was also observed, but thefish recovered after the exposure. Liet al.(2009) also reported on changes ingills and intestine of adult fish. Another study – not peer reviewed – report onattachment of nZVI particles to the outer surfaces of D. magna and indicatesthat EC50 values will be in the mg/L range (EC50, 48 hours of 55 mg/L).(Oberdörsteret al.,2006). In the same study, it was found that exposure offathead minnow to 50 mg/L nZVI did not cause any mortality (Oberdörsteretal.,2006).No studies on the bioaccumulation of nZVI have been found as of 22 October2010. By definition metal nanoparticles are not degradable. Oxidation of Fe0is very likely to occur in aquatic media under normal conditions forming iron-hydroxides. Therefore, nZVI particles cannot be regarded as persistent in thesense that the particles will remain as the original nZVI particles entering theaqueous media. However, a number of environmental factors (like pH, ionicstrength, natural organic matter) may influence the particle sizes and agglom-erate structures in the aquatic environment (Baaloushaet al.,2008; Baalou-sha, 2009) and also nanoparticle characteristics will influence the behaviour.Thus, Schricket al.(2004) showed that unsupported iron nanoparticles willagglomerate rapidly when added to water, but also that different coatings ofthe iron nanoparticles (e.g. anionic hydrophilic carbon, and polyacrylic acid)resulted in colloidal suspensions with slow settling rates (hours to days).5.5Toxicological profile
Only a very limited number of peer-reviewed and published studies on thetoxicological effects of nZVI exist. However, there is evidence that nZVI mayattach to cells and to cause histological and morphological changes (Griegeretal.,2010). Furthermore, some coatings have been found to decrease toxicityprobably due to reduced adherence. The effects observed seem to be linked tothe release of Fe(II) from nZVI followed by production of reactive oxygenspecies. This may lead to disruption of cell membranes and cell death. It hasfurthermore been found that the aging of nZVI (i.e., oxidation of Fe0) re-duces the toxicity of nZVI (Griegeret al.,2010). Thus, the study by Phenratet al.(2009) of the neurotoxic effects of nZVI, “aged” nZVI (oxidized), mag-netite nanoparticles, and surface-modified nZVI, found that “pristine” nZVIwas the most toxic to rodent neuron and microglia cells. The authors foundthat this could largely be attributed to oxidative stress. Mitochondrial swell-ings as well as apoptosis were found when the cells were exposed to nZVI.While the surface-modified nZVI was found to be less toxic than the “pris-tine” nZVI, the coatings were found to potentially facilitate cytoplasmic and
60
nuclear cell entry. Keenanet al.(2009) studied the effects of nZVI on humanbronchial epithelial cells and found that cell viability was affected negativelypossibly due to an internal ROS production. Similar to other studies, ageingof the nZVI resulted in decreased cytotoxicity.5.6Exposure scenarios
The use nZVI involves a direct injection of nZVI nanoparticles in contami-nated ground water through drilled bore holes. In this way clean-up of hard-to-reach sites is made possible (Griegeret al.,2010). In the injections concen-trations 10 g/L of nZVI are typically used (Griegeret al.,2010). The reactiv-ity of nZVI has been estimated to last 4 - 8 weeks, but this does of course de-pend on the specific characteristics of the particles and surrounding environ-mental conditions (like pH, redox conditions and ionic strength).Environmental exposure conditions are largely unknown to day, althoughthere some data is emerging (Griegeret al.,2010). In general, environmentalmigration of uncoated nZVI has been estimated to be only a few centimeters(Tratnyek and Johnson 2006; Salehet al.2008) as a result of aggrega-tion/agglomeration and deposition to other surfaces in the environment(Lowry and Casman 2009). Environmental organisms that may be exposed tonZVI include bacteria, protozoa, and fungi, as well as other unicellular organ-isms if nZVI migrates to surface waters. During and after injection, soil-dwelling organisms may also be exposed to nZVI around injection sites, andhigher plants may potentially be exposed through extensive root systemsreaching groundwater tables (Imadaet al.2008). Although iron occurs natu-rally in the environment, the fate and behaviour of the engineered nZVI maybe different from the naturally occurring iron (Griegeret al.,2010). However,numerous highly complex environmental interactions may occur upon releaseand most of the processes involved in estimating environmental exposures fornZVI are largely unknown today (Griegeret al.,2010).As use of nZVI is only identified in in occupational settings the only very lowexposure portential for consumers is if particles end up in drinking water.5.75.7.1Risk profileEnvironment
As the current use or zero valent iron is very much restricted to gound waterremediation, traditional risk assessment based on toxicity to fish, algae adcrustaeceans is of less relevance for his exposure scenario.However, for nZVI very few ecotoxicological studies have been performed. Itappears that the acute toxicity to aquatic organisms is relatively low, but sub-lethal effects have been observed at concentrations less than 1 mg/L. The ef-fects observed of nZVI in bacteria, under anoxic conditions, may be linked therelease of Fe(II) and subsequent formation of reactive oxygen species. Underaerobic conditions nZVI toxicity is expected to be lower, due to a rapid oxida-tion of Fe0 to iron hydroxides (rust). At present the database is too limited tojustify estimation of PNEC values.Even though environmental exposure concentrations may be high at the siteof injection, transformation and low transport ability seem to limit the risk of
61
exposure to surface waters. High concentrations in soil may occur as a resultof spill during injection. For the time being PEC estimations are hampered bylack of knowledge on the environmental fate and behaviour of nZVI.5.7.2Human health
With the existing applications of nZVI for professional uses only and therebyinsignificant risk of exposure of consumers, the overall risk for consumers isexpected to be low.5.8Summary sheet for nano-scale zero-valent iron
Nanomaterial characteristicsNameNano-scale zero-valent iron(nZVI)8053-60-9FeSource:
CAS numberChemicaltionAppearanceManufacturing proc-essescomposi-
Dark powder
US EPA1)
A number of different synthesis methods exist including the Sol-gelmethods and the sodium borhydride method.
Nanomaterial descriptionnZVI is the nanoform of zero-valent iron. In its most basic form it consists of spherical iron (Fe0)nanoparticles with individual particle dimensions less than 100 nm. Substantial variations exist inthe properties of nZVI in regard to average particle size, particle size distribution, specific surfacearea, surface charge and the presence of trace metals. nZVI may furthermore often be coated inorder to prevent agglomeration and better control their reactivity and mobility. Polymers, polyelec-trolytes, and surfactants are among the main types of coatings used for nZVI.ApplicationsnZVI has been used as a in-situ remediation technology for contaminated sites (e.g. for degrada-tion of PCBs, chlorinated organic solvents, and organochlorine pesticides). A few medical applica-tions have also been reported, but it is the use in soil and groundwater remediation that is antici-pated to be the major use of nZVI in the future.Human health risk profileOnly a very limited number of peer-reviewed and published studies on the toxicological effects ofnZVI exists, however there is evidence that nZVI may attach to cells and to cause histological andmorphological changes. Furthermore, some coatings have been found to decrease toxicity proba-bly due to reduced adherence. The effects observed seem to be linked to the release of Fe(II) fromnZVI followed by production of reactive oxygen species. This may lead to disruption of cell mem-branes and cell death. It has furthermore been found that the aging of nZVI (i.e., oxidation of Fe0)reduces the toxicity of nZVI (Griegeret al., 2010). It is anticipated that there is a very small risk ofhuman exposure to nZVI used for environmental remediation purposes when personal protectionequipment appropriate for handling powders is used. Thus, the overall risk for human health ef-fects is expected to be very low.
62
Environmental risk profileFor nZVI very few ecotoxicological studies have been performed. It appears that the acute toxicityto aquatic organisms is relatively low, but sub-lethal effects have been observed concentrationsthan 1 mg/L. The effects observed of nZVI in bacteria, under anoxic conditions, may be linked therelease of Fe(II) and subsequent formation of reactive oxygen species. Under aerobic conditionsnZVI toxicity is expected to be lower due to a rapid oxidation of Fe0 to iron hydroxides (rust). Atpresent the database is too limited to justify estimation of PNEC values. Even though environ-mental exposure concentrations may be high at the site of injection, transformation and lowtransport ability seem to limit the risk of exposure to surface waters. High concentrations in soilmay occur as a result of spill during injection. For the time being PEC estimations are hamperedby lack of knowledge on the environmental fate and behaviour of nZVI.1)
Http://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/2172/report/2002
63
64
6 Cerium dioxide - CeO26.1General characteristics
Cerium(IV) oxide (CeO2) is an oxide of the element cerium. The crystal formof CeO2is cerianite and according to Wakefieldet al.(2008) CeO2is a ce-ramic compound, which means that it is inorganic and non-metallic. CeO2iscommercially available in a number of different size ranges below 100 nm(Nanowerk 2010).6.2Manufacturing processes
It is not clear at this point in time how the nanoform of CeO2is manufactured,but industrial bulk cerium is extracted from mined minerals, primarily mona-zite and bastnasite (Lide, 2009) whereas CeO2are formed by thermal treat-ment processes.6.36.3.1UsesMain applications
CeO2has several uses exploiting the catalytic ability of CeO2to adsorb andrelease oxygen (Deshpandeet al.,2005) which can be used to coat the insideof self-cleaning ovens, in solar panels and for hydrogen production (Charvinet al.,2009), in fuel cells and in automobile catalysts (Lide, 2009). CeO2hasalso been used as a fuel additive (Wakefieldet al.,2008) and this use may beparticularly important, since it may lead direct emissions during the use phase.CeO2can also be used in polishing agents for glass mirrors (Prospect, 2010).It is not clear whether such uses are deployed in Denmark, however no infor-mation indicating that this is the case has been found. The production and useof CeO2nanoparticles (CeO2-NP) is rapidly growing, and as mentioned aboveCeO2-NP have a number of applications of which the use as a diesel additivein the UK is among the most prominent ones (Rothen-Rutishauseret al.,2009; Wakefieldet al.,2008). For this application CeO2-NP is mixed com-pletely with the diesel (concentration: 5-8 ppm; average particle size: 8-10nm) (Wakefieldet al.,2008). The advantage of using CeO2-NP as a fuel cata-lyst is the improved engine combustion efficiency that results in reduced emis-sions of soot, CO and NOx. Furthermore, the fuel efficiency has been re-ported to increase by 8-9 % (Wakefieldet al.,2008). While Boxall et al (2007)mentions the CeO2is used as a fuel additive in countries like the Philippines,New Zealand and the UK, the amounts used are at present unknown.6.3.2Results from industry survey
The survey has been focused on the use of ceriumdioxide as additive to dieseloil. In this context the trade association for oil products in Denmark has beencontacted. The association informs that ceriumdioxide is not used as additivein oil products by any of the oil companies operating in Denmark (EOF,2010). No information is available on products supplied by smaller companiesoperating on the market in Denmark. Based on the information available it is
65
not possible to quantify the use of ceriumdioxide in Denmark. However, amajor release from fuel is not expected in Denmark.6.4Eco-toxicological profile
For the following overview of the ecotoxicological profile of nano-scale ceriu-moxide (CeO2) it should be stressed that only three ecotoxicological studieswere found and that only one of these found effects of CeO2.Harperet al.(2008) investigated the toxicity of various metal oxides towardsDanio rerio embryos and among these was CeO2, however no effects werereported following exposure to up to 250 mg/L for 5 days.Velzeboeret al.(2008) observed no measurable effects on neither the crusta-cean Chydorus sphaericus nor the freshwater algae P. Subcapitata exposed toa concentration of 100 mg/L CeO2 nanoparticles and tap water for 48 hoursVan Hoeckeet al.(2009) studied the toxicity of CeO2NPs of three differentsizes (14, 20, and 29 nm) towards green algae (Pseudokirchneriella subcapi-tata), two crustaceans species (Daphnia magna and Thamnocephalus platyu-rus), and zebra fish embryos (Danio rerio). They found no acute toxicity forthe two crustaceans and fish embryos in test concentrations up to 1000, 5000,and 200 mg/L, respectively. For the freshwater green algae, EC10-values be-tween 2.6 and 5.4 mg/L were observed.No studies on bioaccumulation of CeO2have been found as of 22 October2010.By definition CeO2nanoparticles are not degradable and speciation of CeO2(e.g. dissociation or complexation) is not likely to occur in aquatic media un-der normal conditions. Therefore, CeO2particles can be regarded as persis-tent in the sense that the particles will remain CeO2-particles, however thesizes of the particles and hence their bioavailability may change due to ag-glomeration and aggregation in aquatic media.6.5Toxicological profile
Very few toxicological studies have been performed on nano-sized CeO2, andall studies performed werein vitrostudies.6.5.1Uptake of CeO2 into cells
No ADME studies has been performed on cerium dioxide, but a study hasbeen performed on lung epithelial cellsThe physico-chemical properties of particles have been illustrated to influenceCeO2uptake and adsorption onto proteins by Patilet al.(2007). The surfacecharge of particles was observed to influence the adsorption of proteins ontothe particle surface. Specifically, BSA adsorbed onto the surface of positivelycharged CeO2to the greater extent, when compared to negatively chargedCeO2. Negatively charged nanoparticles were internalised by A549 cells to agreater extent. Electrostatic interactions were suggested to be the main driv-ing force for the protein adsorption and cellular uptake of CeO2nanoparticles.Surface charge therefore appears to be a determining factor to particle uptakeand protein adsorption.
66
In general the uptake of metal oxides by a variety of cell types has been dem-onstrated on numerous occasions. The consequences of particle uptake intothe cells are assumed to be oxidative stress followed by cytotoxicity and possi-bly genotoxicity. The physico-chemical properties of particles may influencetheir internalisation by cells; accordingly the importance of size and surfacecharge has the ability to influence particle uptake. In addition, the cell typeunder investigation has the potential to determine the mechanism of uptakeand intracellular fate of particles.6.5.2
In vitro
toxicity- lung modelsPulmonary toxicity has been investigated in two different models: rat lungslices and a lung epithelial cell line.CeO2is used as a fuel additive to reduce particulate matter emissions fromdiesel engines. Fallet al.(2007) demonstrated that the CeO2content of dieselexhaust fumes was unable to impact on the toxicity of CeO2supplementeddiesel, within rat lung slices. On exposure of lung slices to freshly generatedfumes, No alterations in cell viability, pro-inflammatory mediator expression(TNFα) and antioxidant enzyme activity (glutathione peroxidase, superoxidedismutase) were identified. However, it was demonstrated that the antioxidantenzyme catalase, and GSH were elevated, at low concentrations of CeO2,which is likely to derive as a defence response. Overall, it was concluded thatCeO2could be tolerated at a high dose. In contrast, exposure of lung slices tonon-supplemented fuel was able to moderately decrease ATP content, anddecrease GSH levels. Therefore, it is implied that the addition ofCeO2introduces a protective effect.Linet al.(2006) investigated the effects of CeO2(20 nm diameter) on theA549 epithelial cell line. Cells were exposed to CeO2at concentrations of upto 23 �g ml-1 for up to 72 hours. A concentration and time dependent in-crease in cytotoxicity and ROS production, antioxidant (GSH and -tocopherol) depletion and lipid peroxidation (indicated by malondialdehyde)were observed. This study therefore highlighted the oxidative and cytotoxicbehaviour of CeO2. Similarly, in a study by Parket al.2008a the cytotoxicityand oxidative potential of CeO2nanoparticles (15, 25, 35, 40 nm) to BEAS-2B epithelial cells was investigated, at concentrations up to 40 �g ml-1, for aperiod of up to 96 hours. CeO2elicited a dose and time dependent decreasein cell viability within BEAS-2B cells, with all particle sizes eliciting a similarresponse. Cytosolic caspase 3 activation and chromosome condensation werealso observed, suggesting that an apoptotic mode of cell death was involved.CeO2exposure was also associated with enhanced cellular ROS production,and a decrease in cellular GSH, so that the cytotoxic response was assumed tobe driven by oxidative stress. No cytotoxic response was observed withinH9C2 cardiomyocyte or T98G brain fibroblast cells, indicating that some celltypes may be more sensitive to the effects of CeO2.6.5.3Dermal models
Parket al.(2007) investigated the toxicity of nano (9 nm) and micro (320nm) forms of CeO2, to the skin,in vitro.The EpiDerm skin model indicatedthat both forms of CeO2tested were skin irritants. No cytotoxicity was ob-served and CeO2was not mutagenic in the Ames test. The toxicity observedwithin this study was not size dependant.
67
6.5.4
Mechanistic studies - Oxidative stress
Oxidative responses exhibited by metal oxide particles have been a focus of anumber of studies, especially for TiO2, and its recurrence has prompted thesuggestion that oxidative stress drives the inflammatory and cytotoxic re-sponses evident. In contrast, a number of investigations have suggested thatcerium dioxide exhibit anti-oxidant, cytoprotective properties as shown belowXiaet al.(2008) investigated whether ZnO (13 nm), TiO2(11 nm) and CeO2(8 nm) oxidative stress development or particle dissolution contributed totheir toxicity. RAW 264.7 macrophages and BEAS-2B epithelial cells wereexposed to the nanoparticle panel for up to 15 hours, at concentrations up to60 �g ml-1. Only ZnO particles were capable of inducing cytotoxicity, withinboth cell types. In addition, ZnO stimulated increased ROS production,which in turn, increased HO-1 expression, and activated the JNK signallingpathway, which paralleled the release of IL-8 and TNFα. Increased calciumrelease was also mediated by ZnO, and was associated with mitochondrialdamage. These findings therefore imply that ZnO elicits an oxidant drivenresponse that was responsible for the initiation of an inflammatory and cyto-toxic response. On the contrary, CeO2was able to stimulate a cytoprotectiveresponse, whereby pre-treatment of cells with CeO2protected against dieselexhaust particle mediated cell damage (which is known to be oxidant driven).All particle types were internalised by an endocytic mechanism, however, onlyZnO accumulated within lysosomes, which was suggested to drive their abilityto inflict oxidative injury, and promote particle dissolution. ZnO also inducedultrastructural alterations, including nuclear fragmentation, apoptotic bodyformation and mitochondrial disappearance. ZnO was therefore recognisedas being the most toxic particle within the panel, however this was thought tobe accounted for, in part, by their dissolution and therefore release of Zn2+ions. The ability of ZnO particles to generate ROS, oxidant injury, inflamma-tion and cell death was demonstrated within this study, highlighting how theseprocesses are inherently linked. The toxicity of the metal oxide particles wastherefore suggested to be driven by their oxidant properties, which was de-pendent on their composition, dissolution and intracellular fate. In contrastCeO2may exhibit cytoprotective response, suggesting that composition playsan important role in determining cellular responses to nanoparticles.Neurones are particularly prone to oxidative stress (Daset al.2007). As a re-sult, Daset al.(2007) investigated the ability of CeO2particles (3-5 nm) toexhibit neuroprotective effects, during the culture of rat spinal cord neurones.The survival of neurones was promoted by the inclusion of CeO2(10 nM)within the cell culture medium, and this was thought to derive from the abilityof CeO2to scavenge free radicals, and thereby prevent against oxidative stress.The antioxidant behaviour of CeO2was also suggested by the findings thatCeO2treatment improved neurone viability following H2O2exposure. How-ever, further studies would be required to confirm this hypothesis.In line with these findings, Schubertet al.(2006) investigated the neuropro-tective behaviour of CeO2from oxidative stress mediated cell death. TheHT22 neuronal cell line was exposed to CeO2nanoparticles (6 and 12 nm) ormicroparticles (1 �m) for 20 hours, at concentrations up to 100 �g ml-1, andno detrimental impact on cell viability was observed. Pre-treatment withCeO2particles were able to protect against glutamate mediated cell death,which is known to be driven by oxidative stress. As a consequence, it was sug-gested that CeO2particles acted as antioxidants, and that this property wasnot size dependent. This was confirmed by the finding that glutamate medi-
68
ated increases in ROS production were diminished by CeO2pre-treatment.The findings were also replicated using yttrium oxide particles. Thereforecontrary to the findings that demonstrate CeO2is toxic, this study illustratedthat CeO2may also exhibit antioxidant, cytoprotective properties.6.5.5Summary
Noin vivostudies have been identified on cerium dioxide, and therefore norisk characterisation can be performed on this substance.Fromin vitrostudies it is evident that surface charge appears to be a deter-mining factor to particle uptake and protein adsorption.There was indication fromin vitrostudies with rat lung slices, that ceriumdioxide had low toxicity and even had protective effect against oxidative stressproduced by diesel exhaust particlesContrary in the lung epithelial cell line A549 CeO2induced oxidative stressand cytotoxicity. No cytotoxic response was observed within H9C2 cardio-myocyte or T98G brain fibroblast cells, indicating that some cell types may bemore sensitive to the effects of CeO2.6.6Exposure scenarios
The use of cerium dioxide nanoparticles as a fuel additive may lead to a directexposure of the general population through inhalation. Boxall et al (2008) es-timated an air concentration a distance of 5 m from roads of 0.6 ng/m3 andParket al.(2008) estimated worst case concentrations of 5-25 ng/m3. Thesevalues are considered to be low when compared to occupational exposureconcentrations (Stoneet al.,2010). For water and soil extremely low pre-dicted environmental concentrations were estimated by Boxallet al.(2008),but it should be emphasised that the data and assumptions used are difficultto validate.6.7Risk profile
The only study on the ecotoxicity of CeO2NPs showed no acute toxicity forcrustaceans and fish embryos in test concentrations up to 1000, 5000, and200 mg/L, respectively. For the freshwater green algae effects were seen at10% inhibition CeO2at concentrations between 2.6 and 5.4 mg/L for threedifferent sizes of CeO2NP. This indicates that CeO2nanoparticles in theworst case can be classified as “Aquatic Chronic 2”, but more likely should beclassified as “Aquatic Chronic 3”.No studies of bioaccumulation of CeO2have been found as of 22 October2010. CeO2particles can be regarded as persistent in the sense that the parti-cles will remain CeO2-particles; however the sizes of the particles may changedue to agglomeration and aggregation in the environment.With only one ecotoxicity study and very uncertain concentration estimates athand, neither PNEC nor PEC can be estimated and therefore no quantitativerisk characterisation can be carried out.Current results do not indicate that CeO2added to diesel fuel increases therisk associated with inhalation of diesel exhaust.
69
6.8
Summary sheet for CeO2
Nanomaterial characteristicsNameCAS numberChemical compositionAppearanceCeriumdioxide, ceriumoxide1306-38-3CeO2Pale yellow-white powderSource: wikipediaManufacturingessesproc-Industrial bulk cerium is extracted from mined minerals, primarilymonazite and bastnasite and CeO2is formed by thermal treatment proc-esses
Nanomaterial descriptionCerium(IV)oxide (CeO2) is an oxide of the element cerium. The crystal form of CeO2is cerianite.CeO2is a ceramic compound, which means that it is inorganic and non-metal. CeO2is commer-cially available in a number of different size ranges below 100 nm.ApplicationsCeO2has several applications and due the catalytic ability of CeO2to adsorb and release oxygen itis used e.g. to coat the inside of self-cleaning ovens and for hydrogen production in fuel cells. Themost widespread use of CeO2is as an additive to diesel. This use may be particularly importantfrom an environmental point of view, since it may lead direct emissions during the use phase. Forthis application CeO2-NP is mixed completely with the diesel (concentration: 5-8 ppm; averageparticle size: 8-10 nm). The advantage of using CeO2-NP as a fuel catalyst is the improved enginecombustion efficiency that results in reduced emissions of soot, CO and NOx. Furthermore, thefuel efficiency has been reported to increase by 8-9 %. The production and use of CeO2nanoparti-cles (CeO2-NP) is rapidly growing and CeO2 is used as a fuel additive in countries like the Philip-pines, New Zealand and the UK. However, the amounts produced and used are at present un-known.Human health risk profileNoin vivostudies have been identified on cerium dioxide, and therefore no risk characterisationcan be performed on this substance.Fromin vitrostudies it is evident that surface charge appears to be a determining factor to particleuptake and protein adsorption. There was indication fromin vitrostudies with rat lung slices,that cerium dioxide had low toxicity and even had protective effect against oxidative stress pro-duced by diesel exhaust particles.Contrary in the lung epithelial cell line A549 CeO2 induced oxidative stress and cytotoxicity. Nocytotoxic response was observed within H9C2 cardiomyocyte or T98G brain fibroblast cells, indi-cating that some cell types may be more sensitive to the effects of CeO2.
70
Environmental risk profileThe only study on the ecotoxicity of CeO2NPs showed no acute toxicity for crustaceans and fishembryos in test concentrations up to 1000, 5000, and 200 mg/L, respectively. For the freshwatergreen algae effects were seen at 10% inhibition occurred at concentrations between 2.6 and 5.4mg/L for three different sizes of CeO2NP. This indicates that CeO2nanoparticles in the worst casecan be classified as “Aquatic Chronic 2”, but more likely should be classified as “Aquatic Chronic3”. No studies of bioaccumulation of CeO2have been found as of 22 October 2010. CeO2particlescan be regarded as persistent in the sense that the particles will remain CeO2-particles; howeverthe sizes of the particles may change due to agglomeration and aggregation in the environment.With only one ecotoxicity study and very uncertain concentration estimates at hand, neither PNECnor PEC can be estimated and therefore no quantitative risk characterisation can be carried out.
71
72
77.1
Silver - AgGeneral characteristics
Nanosilver is the nanoform of silver characterized by being spherical particlesof a size ranging from 1-250 nm. Nanosilver is commercialized as powder,flakes, grains, ingots, etc., and is sold in suspension (in water, alcohol or sur-factant) and as a dry powder. Nanosilver is furthermore available in prepara-tions (e.g. as a coating agent, in alloys, etc.) and in articles (electrodomesticappliances, in textiles, in food packages, etc.). In its pure form nanosilver willaggregate and hence nanosilver is often surface modified with for instancedextran, citrate, polysaccharide, hydrocarbon or polyvinylpyrrolidone (PVP).Sometimes nanosilver is also found to be deposited on or used as a coating ofa substrate such as plastic, silica or polymers, to give a desired adhesion, elec-trical conductivity, etc. (Luomaet al.2007, Nanowerk 2010, Pronket al.2009). In aqueous solutions nanosilver forms dissolved free silver ions inaqueous by dissolution and subsequent oxidation.7.2Manufacturing processes
Nanosilver can be produced through a number of methods such as ultra-sonicprecipitation and chemical vapour deposition, but also exploding wire synthe-sis. By using silver salts as a starting material and then adding various surfaceactive agents and coatings, the size, shape, surface area, etc. can be modified.Nanosilver sold in suspensions needs to be surface-modified or suspendedwith a chelator (e.g. citrate) to ensure the stability of the nano-silver particlesin suspension.7.37.3.1UsesMain applications
The use of nanosilver is very diverse and includes therapeutic applicationssuch as wound dressing personal care products, powdered colours, varnish,textile, paper, interior and exterior paints, printing colours, water and air-purification, polymer-based products and foils for antibacterial protectionsuch as washing machines. The main consumer uses seems to be linked to theantibacterial properties of silver such as clothing for preventing odour (e.g.socks, underwear, sport textiles) toothpaste and self-sanitizing toothbrushes.Potential usage of nanosilver could be Food Contact Materials (FCM) likekitchenware and containers for food storage and even as food supplement.However, the uses of silver on nanoform for food related purposes (i.e. asFCM or food additives/supplements) require more data on toxicity and expo-sure in order to get approval for such uses in EuropeHowever, nanosilver can be bought on the internet as colloidal silver in foodsupplements for treating certain diseases and as allergy prophylaxis (Gulbran-sonet al.2000; Silver 2003).
73
Nowacket al.(2011) underlines that products containing nanosilver particleshave been commercially available for decades in diverse applications, e.g. inbiocides and without use of the prefix "nano". In the United States all silverregistrations with EPA from 1970 to 1993 were for nanosilver (colloidal sil-ver) or for silver nanocomposites. EPA has also registered several biocidaladditives based on elemental silver particles with particle size < 100 nm.Today silver is being used in many applications due to a desire to shift awayfrom organic chemical agents toward additives, which can be used in muchlower concentrations in a wider variety of products including applications re-quiring high temperature processing which is not feasible for organic com-pounds (Nowack et al, 2011).On the Danish market a total of 178 products have been identified to becommercially available through the internet. Of these which 111 products fallinto the category of Health and Fitness. 17 and 16 products fall into the cate-gory of Home & Garden and Food & Beverages, respectively. In the categoryof Health and Fitness the large majority of the products (69) fall into the cate-gory of personal care products whereas a smaller minority of the products fallsinto the categories of Filtration (19) and Clothing (12) and Sporting goods(8). These numbers should be seen as indicative numbers only since a num-ber of methodological concerns may introduce a bias in the actual numbers ofproducts in the market (as described in section 2.2.1).In the cases of shampoo, soap and toothpaste the concentration of nanosilverhas been reported be 0.001-0.002 % (Boxallet al.2008), but in general thescale of use nano-silver in terms of percentages in consumer products is un-known at this point in time.7.3.2Results from industry survey
Regarding the use as biocide in clothes request for information has been madeto dominant international suppliers of sports equipment. While some compa-nies Nike (Nike 2010; Intersport 2010) informs that nanosilver is not used insports equipment, other companies e.g. Adidas has not responded. Overall itcan be concluded that no confirmation of the use of nanosilver particles in thiscontext has been received, but indications exist that some products/brandsmay contain nanosilver. A manufacturer of nano silver yarn presents the fieldsof application as active/casual/sports/outdoor wear, under wear and home fur-nishing and bedding (Everest 2010).Quantification of the amount of nanosilver used for this purpose is not possi-ble.7.4Eco-toxicological profile
A number of ecotoxicity studies on AgNP has been reported in the literatureand it seems that the number of papers on AgNP is rapidly increasing. This isprobably due to the inherent toxicity of the element silver and many studiesaim to disclose the difference between nano-scale Ag and the Ag+-ion. It isbeyond the scope of this report, to give a full review of the ecotoxicity of Agor AgNP. For this the review by Ratte (1999) and Weijnhovenet al.(2009)give good overviews. Here the focus is on the studies that are considered to berelevant for e.g. hazard classification or the predicted no-effect concentrationfor AgNP
74
For fish a 48 hour static toxicity tests on adult zebrafish (Danio rerio) re-vealed LC50, 48h of 7.07 (6.04-8.28) mg/L (Griffittet al.,2008) and 7.20(5.9-8.6) mg/L(Smithet al.,2007). Using zebra fish embryos decreased dose-dependent hatching rates, weak heart beats, edema and abnormal notochordshas been reported by Yeo and Kang (2008) after 48 hours exposure of 0.01mg/L and 0.02 mg/L 10-20 nm Ag nanoparticles suspended in tap water.A number of short-term studies have been performed on Ag nanoparticles onpelagic crustaceans and a number of LC50, NOEC and LOEC have beenderived. 48 hour EC50 values have also been derived from a short-term toxic-ity testing on adult Daphnia pulex and Ceriodaphnia dubia neonates and re-ported LC50, 48 h to be 0.040 (0.030-0.050) mg/L and 0.067 mg/L (Griffittet al.2008), respectively. In the same study an EC50 0.19 mg l-1 after 96hours was found for green algae (P. subcapitata). For another alga species(Chlamydomonas reinhardtii) EC50 ranged from 0.355 mg/L � 0.062 mg/Lafter 1 hour, to around 0.092 � 0.011 mg/L after 3-5 hours. Expressed as afunction of free Ag+, EC50 was estimated to range from 3.6 � 0.5 ug/l after 1hour, to 0.9 � 0.08 ug/l after 5 hours (Navarroet al.2008). This study is im-portant because it was found that the toxicity of AgNP cannot solely be ex-plained by the free ion (Ag+) (Navarroet al.2008).The widespread use of AgNP as a disinfectant in consumer products raisesthe questions whether unwanted environmental side-effects can occur. In thisrespect, the effect of AgNP on the biomass in wastewater treatment plants isespecially important. In studies of effects on nitrifying bacteria Choi and Hu(2008) and Choiet al.(2008) used microbial growth inhibition tests to studythe effect of different sizes (9-21 nm) of Ag nanoparticles in concentrations of0.05-1 mg/L. At exposure concentrations of 1 mg/L a significant inhibition of86 � 3% was observed for Ag nanoparticles compared to 42 � 7%, and 46 �4% for Ag+ ions and AgCl colloids, respectively (Choiet al.2008). No corre-lation between nanoparticle size and bacteria inhibition in respirometry tests.However, a correlation was found between inhibition and the fraction ofnanoparticles with sizes less than 5 nm, indicating that this fraction mighthave a stronger effect on respiration than larger size fractions. Furthermore itwas found that, at the same total concentration of Ag, Ag nanoparticlescaused a greater inhibition than the free Ag+.By definition metal and metal oxide nanoparticles are not degradable. How-ever, changes in the silver speciation can occur depending on redox condi-tions, salt content etc. For ionic silver the speciation is the determining factorfor bioavailability and ecotoxicity. Though the speciation of silver most likelywill play a major role in the ecotoxicity of AgNP (in analogy to the case ofionic silver), the changes in speciation of the elemental silver are poorlydocumented and no general conclusion can be made in this regard (Stoneetal.2010).7.5Toxicological profile
The toxicological studies on nano-silver described below are quoted fromStoneet al.(2010), Christensen et al 2010 and Wijnhoven et al, 2009. I addi-tion a few recent papers from the open literature are included.The toxicity of silver metal (bulk) and silver compounds is not described indetail in this report, and only a few studies on bulk silver are available com-pared to nano-silver and dissolved silver. (Nowack et al 2011). The toxicity of
75
silver is considered to be relatively low (Wijnhoven et al, 2009), and minimalrisk is expected due to clinical exposure by inhalation, ingestion, dermal appli-cation or through the urological or haematogenous route. Toxicity studies onsilver are mainly human studies and toxic effect on humans other than agyriahave only been observed at very high concentrations. Chronic ingestion orinhalation of silver preparations (especially colloidal silver) can lead to deposi-tion of silver metal/silver sulphide particles in the skin (argyria), eye (argyro-sis) and other organs. These are not life-threatening conditions but cosmeti-cally undesirable and irreversible. The most dramatic symptom of argyria isthat the skin becomes blue or bluish-grey colored (ASTDR). Agyria is mainlycaused by medical use of colloidal silver. The estimated total dose required toinduce argyria by ingestion is in the range of 1-30 g for soluble silver salts(Nordberg and Gerhardsson, 1988). Already in 1939 a threshold value abovewhich argyria can be expected was set (Nowack et al 2011). This value wasset to 0.9 g of silver over the whole lifetime. Later (WHO / SDE / WSH /03.04 / 14) a total lifetime oral intake of about 10 g of silver was considered asthe human NOAEL on the basis of present epidemiological and pharmacoki-netic knowledge, This value corresponds to a daily intake over 70 years of 0,4mg/person. In Denmark the limit value for silver in drinking water is set to40.01 mg/L corresponding to 2.5% of the human NOAEL.7.5.1ADME studies
AbsorptionInhalation63After exposure to nano-silver (4-10 nm) 3 x 10 particles cm- equivalent to-3133�gm for 6 hours silver was deposited in the lung. Silver was measured inthe blood and low concentrations of silver were found in the liver, kidney,spleen, brain and heart. It was not clear whether the absorbed silver includedsilver nanoparticles, free silver ions (Ag+), a silver complex or a combination(Takenaka et al (2001).In a 90 day whole body inhalation study to silver nanoparticles (18-19 nm), atlow, medium and high doses, deposition of silver in the lung was observed -as well as silver in the blood and secondary organs such as liver, olfactorybulb, brain and kidneys Sunget al.(2008, 2009). Also this study did not clar-ify whether the absorbed silver was nano-silver or free ions. A similar distri-bution pattern was observed in a 28 day inhalation study (Jiet al.(2007)).OralAbsorption via the oral route was investigated in a 28 days study with expo-sure to very high doses (up to 1000 mg/kg bw/day) compared to what wouldbe seen in an occupational or consumer situation. Deposition of silver wasobserved in different organs (brain, liver, kidneys, lungs and testes) indicatingtransfer of silver into blood (Kimet al.(2008).There is also indication that silver can be transported to the skin after oral ex-posure, where blue-grey colouring of the skin (argyria) has been observed(Wadhera and Fung (2005))4
(Bekendtgørelse nr 1449, 2007)
76
As for uptake via the lung, none of the above studies clarify whether the up-take is as nano-silver, silver ions, silver complexes or a combination of thetwo. However, it must be anticipated that at least part of the silver uptake fol-lowing oral exposure to nano-silver takes place as silver ions. This assump-tion is – at least partly – supported by the fact that some of the same toxic ef-fects as seen following non-nano silver exposure are seen following nano-silver exposure (in particular argyria).DermalSome studies have shown that silver nanoparticles can be absorbed via theskin when used in wound dressings. In these studies, the wound dressingswere applied to damaged skin every 2-4 days. Vlachouet al.2007 showed thatserum levels increased with increasing exposure time peaking after 9 days andreturning to normal 6 month after exposure.In anin vitrodiffusion cell systemLareseet al.(2009)investigated the penetra-tions of polyvinylpirrolidone coated silver nanoparticles (25 nm) on intact and-2damaged human skin slices. Silver nanoparticles (70 �g cm , for 24 hours)were able to pass through the skin preparations. The absorption was very low(0.0006% of applied dose with intact skin) but detectable and the penetrationwas 5 times greater in damaged skin. In addition, silver nanoparticles could bedetected by electron microscopy (TEM) in the stratum corneum and the out-ermost surface of the epidermis.As for uptake via the lung, it is not clear whether the uptake via the skin is asnano-silver, silver ions, silver complexes or a combination and the relevanceof thesein vitrofindings needs to be investigatedin vivo.DistributionFollowing absorption silver was seen in the following secondary organs: liver,kidney, spleen, hearth, olfactory bulb, brain, testes and skin (discoloration ofthe skin), with liver being the likely major secondary organ site of accumula-tion. As noted above there is no clear indication whether the absorbed anddistributed silver occurs as silver nanoparticles, silver ions, silver complexes ora combination.MetabolismAs described earlier there is no clear indication whether it is silver nanoparti-cles, silver ions, silver complexes or a combination that is absorbed. There isthus also no clear data suggesting whether and how the silver is further trans-formed once in the body. However, one of the biological/toxic effects seen insome studies (argyria - being a blue-grey dis-colouring of the skin) may, atleast partly, involve a transformation/reduction of silver ions to metallic silverfollowing exposure to UV-radiation/sunlight. This could indirectly suggestthat some of the silver in circulation occurs as silver ions. These ions will atleast partly be detoxified as “normal” silver ions by precipitation as sulphideand chloride salts and binding to proteins making them less bioavailable.However, if nanosilver is absorbed as particles these particles may continu-ously release silver ions within the tissue and thereby, at least partly, bypassthe detoxification mechanism. Therefore nanosilver may be more toxic thanthe non-nanoform.
77
EliminationTropet al.(2006) showed elevated silver levels in the urine following expo-sure. No other data for nano-silver elimination has been identified.7.5.2Oral
Acute toxicity
After a single oral dose of nano- (15 nm) and micro-particles (2 - 3.5�m) sil-ver, ingested directly into the stomach of mice histopathological analysis of theliver tissue, 3 days post-exposure, demonstrated evidence of inflammation inthe form of lymphocyte influx for both particle sizes (greater response for thenanoform). This was further supported by changes in the gene expression of4 genes involved in inflammation (Chaet al.(2008)). This dose seems to berelatively high in relation to what would be expected in terms of occupationaland consumer exposure. The relevance of these findings is therefore dubiousfor the purpose of risk assessment.InhalationNo acute toxicity studies have been identifiedDermalNo studies investigating the dermal acute toxicity of silver nanoparticles onhealthy skin has been identified. However, there are signs of argyria and livertoxicity following treatment of burns with wound dressings. The relevance ofthis data for healthy skin at lower concentrations and longer exposure time (asexpected in occupational and consumer settings) is uncertain. Therefore, fur-ther investigations are needed on healthy skin with doses and exposure timesrelevant for exposures as seen in occupational and consumer settingsOther routesNo studies were identified studying toxicity via other routes of exposure.7.5.3Irritation and corrosion
No studies have been identified investigating specifically the irritating behav-iour of silver nanoparticles (to the eyes, lungs or skin).Silver nanoparticles are not expected to be irritating to skin based on the hu-man findings of applying it in wound dressings. However, further informationwould be needed to draw firm conclusions. Based on the above considera-tions, silver nanoparticles are also not assumed to be corrosive.7.5.4Sensitisation
No studies have been identified investigating the sensitising behaviour of silvernanoparticles. As silver nanoparticle containing wound dressings are routinelyapplied and none of the studies investigating that application have reportedsensitisation, it appears unlikely that nano-silver is a dermal sensitiser. Re-garding possible sensitisation following inhalation, further proof/investigationis required.Based on these results it would be relevant to investigate the toxic effects onorgans where silver was depositied especially on the reproductive system.
78
7.5.5
Repeated dose toxicity
OralIn a 28 day repeated dose toxicity in mice (OECD, 407) by Kimet al.(2008),a dose-dependent toxicity was observed in the liver (histopathology changesand inflammation), insinuating that this organ is a target site for silvernanoparticle toxicity. However, it should be noted that the doses used withinthis study were relatively high (up to 1000 mg/kg bw/day), which should beaddressed if used for a risk assessment. However, due to limited histopa-thological information, no oral No Observed Adverse Effect Level (NOAEL)was established based on these data.Recently a 90 day oral study according to OECD guideline 408 and in com-pliance with GLP has been registered under REACH (EC Regulation no51907/2006ref ). Nanosilver (56 nm) was investigated in male and female wis-tar rats. The animals were dosed daily by gavage with 0, 30, 125 and 500mg/kg bw/day. The doses were based on the 28 days study by Kim et al(2008). The study referred to in REACH was performed by Kim et al (2010).The target organ for the silver nanoparticles was found to be the liver in boththe male and female rats after 90-day of exposure to silver nanoparticles. Sig-nificant dose-related changes were found in alkaline phosphatase and choles-terol levels of male and female rats at and above 125 mg/kg bw/d, indicatingslight liver damage. Histopathology revealed slightly higher incidences of bile-duct hyperplasia with or without necrosis, fibrosis and/or pigmentation intreated animals together with a dose-dependent accumulation of silver in alltissues examined. A NOAEL of 30 mg/kg bw/day and LOAEL of 125 mg/kgbw/day was established based on effect on the liver.There was a statistically significant (p<0.01) dose-related increase of silverdeposition in testes, liver, kidneys, brain, lungs and blood of treated rats. - Atwo-fold higher accumulation of silver was seen in kidneys of females com-pared to males.InhalationIn a 28 days inhalation study rats were exposed to silver nanoparticles (13 –15 nm) in an inhalation chamber 5 days a week and 6 h per day to 3 differentdoses (Hyunet al.(2008)). It was concluded by the authors that although aslight effect was observed on the neutral mucins in the respiratory mucosa, no3significant toxicological effect was observed with exposure up to 61 �g/m .In another 28 days inhalation study (OECD 412) in rats by Jiet al(2007)with the same exposure duration and dose levels as above some toxicity (cy-toplasmic vacuolation and heptatic necrosis) was observed within the liver,but histopathological analysis did not reveal any distinct toxicity within otherorgans.A 90 days whole body inhalation study was performed according to OECDguideline 413 (Sunget al.,2008, 2009). Rats were exposed to silver nanopar-33ticles (18-19 nm), at low (49 �g/m , equivalent to 0.6x106 particles/cm and23331.08x109 nm /cm ), medium (133 �g/m , equivalent to, 1.4x106 particles/cm233and 2.39x109 nm /cm ) and high (515 �g/m , equivalent to 3.0x106 particles /All, non-confident data is publicly available onhttp://apps.echa.europa.eu/registered/registered-sub.aspx5
79
cm and 6.78x109 nm /cm ) doses for 6 h/day and 5 days/week. Lungs andliver were the main target organs for accumulation of silver and toxicity.Prolonged exposure to silver nanoparticles was demonstrated to elicit an in-flammatory response within the lung, and induced alterations in lung func-tion, at all par ticle concentrations (Sunget al.2008), indicating that the low-3est dose (49 �g/m ) would be a lowest observed effect concentration(LOAEC). The silver concentration was observed to increase in the blood,indicating that silver nanoparticles were transferred into blood from the lung,after which they subsequently became distributed within the liver, olfactorybulb, brain and kidneys. However, the silver content within these tissues mayderive from nanoparticle or ion presence, which warrants further investiga-tion. In the Sunget al.(2009) study, which reported on clinical observations,haematology and histopathological examinations, erythrocyte aggregation andkidney function test, toxic effect on liver and lung was observed at the highestconcentration, and a No-observed Adverse Effect concentration (NOAEC) of3100 �g/m was suggested. However, as this dose was not an observed value,3the middle dose of 133 �g/m , the observed dose without significant liver ef-fect, will be applied as the NOAEL. Because it is uncertain whether the lung3effect is an adverse effect, both values (49 and 133 �g/m ) will be used in therisk characterisation.DermalNoinformation identified except the dermal studies reported under acute tox-icity.Other routesNostudies identified7.5.6Mutagenicity
3
2
3
No mutagenicity or genotoxicity studies classically used in chemical regula-tory setting have been identified.A micronucleus test was performed at the end of the 28 days oral study in rats(described under the repeated dose oral toxicity study) (Kimet al.2008). Noclastogenic/aneugenic effect was observed. However, it should be noted thatno positive control was included.Nano-silver may cause mutagenicity/genotoxicity via an indirect thresholdedmechanism driven by formation of ROS. Further testing of the possible directgenotoxicity of nano-silver is needed.7.5.7Carcinogenicity
No studies investigating the carcinogenicity of silver nanoparticles have beenidentified. However, as silver nanoparticles may be genotoxic, carcinogenicitycannot be excluded. However, further chronic carcinogenicity studies shouldawait results on mutagenicity of nanosilver.7.5.8Reproductive toxicity and developmental toxicity
FertilityIn anin vitrostudy on mouse spermatogonial stem cell (SSC) Bradyich-Stolleet al.(2005) invetstigated the cytotoxicity of different nanoparticles (Silver(15 nm), molybdenum trioxide (MoO3, 30 nm), and aluminum (Al, 30 nm))-1using the LDH and MTT assays. The cells were exposed to 10 �g ml for 48
80
hours. Findings demonstrate a concentration-dependent toxicity for all typesof particles tested, whereas the corresponding soluble salts had no significanteffect. Silver nanoparticles were the most toxic.In a later study Bradyich-Stolleet al.,2010 investigated the cytoxicity and cellproliferation of Ag-NPs of different sizes (10, 25-30 and 80 nm) coated witheither polysaccharide (Ag-PS) or hydrocarbon (Ag-HC) in the same cell lineas in the 2005 study above. The results of the 2010 study showed that Ag-NPs reduced the viability and cell proliferation of SSC and that the effect issize and concentration dependant. It was also shown that, over time, there wasno (protective) effect of particle coating. At low exposures decrease in cellproliferation was not due to ROS formation but other mechanism like interac-tion with cell communication. Electronmicroscopy (TEM) showed optake ofAg-NP.These results confirm that toxicity of Ag-NP increase with decreasing particlesizein vitro.Although it has been demonstration fromin vivoinhalation stud-ies (Sunget al.2009) and oral studies (REACH) that nanosilver is distubutedto different organs, including testes, there was no detailed analysis of toxiceffect on testis, but the exposure concentration would have been much lowerwith a blood concentration of 4 ng/g blood, than in thein vitrostudies with-1germ cells where a concentration of 10 �g ml was used Therefore it is diffi-cult to relate thein vitroresults to human exposures, andin vivostudies areneeded to draw a conclusion.No data on female fertility has been identified.Developmental toxicityDevelopmental toxicity was investigated in two embryo fish studies and com-pared with toxicity of gold nanoparticles (Bar-Ilanet al.,2009). It seems thattoxicity (malformation and death) is dose dependent and that silver is consid-erably more toxic than inert gold nanoparticles. As for fertility, further inter-pretation requires better understanding of the toxicokinetics and possibly sub-sequent confirmationin vivo.7.5.9Biological mechanism
A range of mainlyin vitrostudies have shown that the toxicity of silver andother metal nanoparticles appear to be mediated by the induction of inflam-mation and oxidative stress, which can result in cytotoxicity and genotoxicity.7.5.10 SummaryIt has been shown that silver from nanoparticles can be absorbed especiallyvia oral and inhalation routes of exposure. Based onin vitrodata there is indi-cation that dermal absorption occur to a lesser extent, and intact skin seems tobe a rather effective barrier against absorption, howeverin vivodata areneeded for a firm conclusion. However, it is unclear in which form (as parti-cles, free ions, silver ions or complexes) nanosilver is absorbed and distributedto target organs. At least for uptake via the oral route it is likely that at leastsome of the uptake occurs as ions. It appears that smaller particles exhibithigher toxicity as compared to larger particles; and if silver is absorbed as par-ticles then the surface area is relevant.Should silver uptake occur solely as ions, the database for silver could be ap-plied to assess systemic silver nanoparticle toxicity. For that exercise, it would
81
need to be considered whether and how the dramatically increased surfacearea and possibly increased solubility of silver nanoparticles would need to betaken into account.Quite a few, mainlyin vitrostudies, have shown that the main mechanism ofsilver nanoparticle toxicity seems to be mediated by an increase in ROS pro-duction (except toxicity on germ cells), stimulating inflammation andgenotoxic events and apoptosis or necrosis. The concentration of the adminis-tered nanoparticles is able to influence the toxicity, specifically, at low levels ofoxidative stress a protective response is initiated which progresses to a damag-ing response with increasing particle concentration, and therefore oxidant lev-els. It is thus relevant to consider that the (geno)toxicity of silver nanoparticlesis thresholded.7.6Exposure scenarios
Silver is one of the most widely used nanoparticles in consumer products(Wijnhovenet al.,2009) and especially the uses in textiles and personal careproducts may lead to human and environmental exposures.Nano-silver in textiles is used in all kinds of clothes from sock and shirts tocaps, gloves and underwear. In all cases it is the antimicrobial activity of nano-silver that is the reason for incorporating it in textiles. The environmental re-leases from textiles have been investigated in some theoretical studies and afew laboratory based ones. In the study by Luoma (2008) it was estimatedthat mass release from silver containing socks in the USA would be in therange of 6-930 kg or 180-2790 kg assuming that 10% and 30%, espectively, ofthe population would use these kinds of socks. The release of nanosilver fromsocks upon contact with water showed that for some socks almost all silverleached to water whereas for others no leaching was detected (Benn &Westerhoff, 2008). In a simulation of the washing of silver-containing textiles,Geranioet al.(2009) found varying percentages of the total silver emittedduring one wash, i.e. from less than 1% and up to 45%. Bennet al.(2010)measured the content of silver in textiles (in a shirt, a medical mask, a toweland a cloth), personal care products (toothpaste, shampoo), a detergent, a toy(teddy bear), and two humidifiers. They found silver concentrations from 1.4–1to 270,000�gAg g product . Upon wasing in tap water they estimated thepotential release of silver into aqueous environmental matrices in quantities upto 45�gAg/g product. By electron microscopy Bennet al.(2010) were ableto confirm that nano-silver particles were present in the products, but also inthe wash water samples.In what can best be characterized as first attempt on material flow analysisMueller and Nowack (2008) made some estimation of predicted environ-mental concentrations of silver and Gottschalket al.(2010) have further re-fined these estimates. For surface water, Gottschalket al.(2010) derive a PECvalues of 0.72 ng/l and for sewage treatment plant effluents a PEC of 11.8ng/l.Human exposure to nanosilver is suspected to be highest in the working envi-ronment. The main exposure route for occupational settings is primarily viainhalation and dermal contact.For consumer exposure no quantitative data have been identified, but it mustbe assumed that the main exposure route is dermal contact for clothes and
82
paint applications, and inhalation for spray applications. Oral exposure mayoccur from toothpaste and toothbrushes containing nanosilver and if, in thefuture, nanosilver will be permitted for FCM and in food supplements (see5.2.1). The magnitude of exposure will depend on the concentration in theconsumer product, the amount released from the products, which depends onhow strong nanosilver is bound in the solid matrix applications (e.g. in clothesand FCMs).7.77.7.1Risk profileEnvironment
Silver is known to be an ecotoxic metal, however the toxicity is highly de-pendent on the form and speciation of the metal. In the registration of silverunder REACH (EC Regulation no 1907/2006), predicted no effect concen-trations (PNECs) are reported to 0.04 �g/L (freshwater), 0.86 mg/L (marinewater) and 0.025 (sewage treatment plants). Test with silver nanoparticles(AgNP) do also reveal very low effect concentrations. Thus, for algae EC50-values a low a 4�g/lhas been found and also for crustaceans values far below1 mg/L have been reported. This ranks AgNP as "Aquatic chronic 1". It is alsoimportant to note that at concentrations below 1 mg/L inhibition of nitrifyingbacteria can occur and thus the function of wastewater treatment plants maybe affected by the presence of AgNP. For ionic silver it is known that thespeciation in aqueous media is determining for the bioavailability and toxicity.This is most likely also the case for elemental silver nanoparticles, but influ-ence of speciation on uptake, depuration, and toxicity remain to be studied inthis case.The environmental concentration resulting from the use of AgNP in con-sumer products are at present uncertain, even though a number of differentestimates have been proposed. It is evident that even though silver nanoparti-cles are incorporated in textiles, they can be released upon washing. Concen-trations in the low ng/l range have been proposed and even such low concen-6trations may under very precautious assumptions constitute an environmentalrisk due to the high toxicity of silver.It is debated today whether silver nanoparticles are in fact more toxic thantheir bulk counterpart, since effects in many cases can be ascribed to the ionicform of silver (Ag+). Some studies have documented a higher effect ofAgNP, but it is the widespread and disperse use of silver in consumer prod-ucts that poses the greatest risk to the aquatic and terrestrial environment.Even if AgNP are “only” as toxic as larger silver particles, silver is still a veryecotoxic metal.7.7.2Human health
Although nano-silver is presumably the most widely applied nanomaterial onthe market, and relatively high exposure to workers and consumers might beexpected, there is still a need for further data on exposure and human toxicityto fill out existing data gaps. Some important areas for research needs arementioned below.
These assumptions are using the highest assessment factor of 1000 and are assumingthat the bioavailability of silver is not reduced due to complexation.
6
83
Human exposure data are very important for a risk characterisation.However, no or very few data on concentrations as well as data on sizeand form (e.g. aggregates, agglomerates) in consumer products exists.Such data are urgently needed in the future, in particular for occupa-tional inhalation and consumer inhalation and dermal exposure. Insuch studies characterisation of the silver nanoparticles in terms ofsize and agglomeration states, as well as a clear indication of durationand frequency of exposure is importantFurther investigations of the nano-silver toxicokinetics. In particular,to which extent the silver absorbed via different routes becomes sys-temically available as ions, as nanoparticles and/or as silver complexes,and whether the size of the silver nanoparticles influences the uptakemechanism(s);Further testing for the possible direct genotoxicity of silver nanoparti-cles; and depending of the results of these studies carcinogenicitystudies might be neededWhen more knowledge is obtained on absorption and distribution ofdifferent sizes of nano-silver is obtained, then effects on other organsthan liver and lung should be investigated in (sub)chronic studies fol-lowing different exposure routes, especially inhalation and dermal ex-posures are relevant for consumersFurtherin vivoinvestigation of the possible reproductive and devel-opmental toxicity of silver nanoparticles
However, some (limited) studies exists on which this (preliminary) risk char-acterisation is basedThe acute toxicity of nanosilver seems to be low for mammals. For solublesilver nitrate the estimated acute lethal dose is at least 10 g for humans.However,if absorbed as particles nanosilver may be more toxic than the non-nano formdue to the small size and larger surface.In vitrogenotoxicity data, indicate that nanosilver may cause mutagenic-ity/genotoxicity via a thresholded mechanism. Therefore a human NOAELcan be established, provided that sufficient data are available.Risk following inhalationDue to uncertainties of the genotoxic potential and the limited dataset in theexisting studies reported so far, only a human Indicative No-effect Levels(INELs) can be set for inhalation.As mentioned earlier and proposed in Stone et al 2010 and Christensenet al.2010 a human no effect level will be based on reduced lung function (LOAEL33of 49�g/m) and systemic liver effect (NOAEL of 133�g/m).Using assessment factors as set out in the REACH guidance (ECHA 2008,chapter R.8) and explained in Stone et al 2010 and Christensenet al.2010 thefollowing INELs were derived:
84
Reduced lung function used as critical effect1. Scenario: using a factor 3 for extrapolation a LOAEL to a NOAELINELlung 1= 0.33�g/mequivalent to 4000 particles m-3 and 7.2 x 10 nm /cm2. Scenario: using a factor 10 for extrapolation a LOAEL to a NAELINELlung 2= 0.098�g/mequivalent to 1200 particles m-3 and 2.2 x 1023nm /cmSystemic liver effect as critical effectINELliver= 0.67�g/mequivalent to 7000 particles m-3 and 1.2 x 10 nm /cm33623363623
These values are much lower as compared to a DNEL value of 0.04 mg/mfor bulk silver for systemic effects, which has been derived by the registrant ofsilver in the REACH registration dossier on silver (ECHA web site April72011)The differences between the INELs and DNEL above clearly indicate thatnano-silver might be more toxic than bulk silver after inhalation.It has been estimated (Christensenet al.2010) that peak occupational expo-sure can be at same order of magnitude or even higher as the above calculatedINELs when expressed in particle number. However, better data (exposureand toxicity data) are needed.No quantitative risk estimation can be carried out for consumers because noinhalation data have been identified for consumers. However, given thatnanosilver applications in spray have been reported, this exposure route couldbe of concern, and the identification of exposure values via inhalation shouldbe prioritised.Based on available toxicological data it is recommended to avoid exposure viainhalation, because health effects like reduced lung function and toxic effecton the liver cannot be excluded after repeated exposure. There is also indica-tions fromin vivoinhalation studies that silver can reach the germ cells andtoxicity to germ cells has been observedin vitrofrom AgNP, although atmuch higher concentrations than could be expected from consumer exposure.Risk following dermal exposureNo quantitative data related to occupational and consumer dermal exposurehas been identified. It must be assumed that especially consumers can be ex-posed to nanosilver due to the relatively widespread use in clothes. Very fewtoxicity data for nanosilver are available, but human data have indicated toxic-ity (agyria and liver toxiciy) after use of wound dressings containingnanosilver. However, consumer exposure is considered to be much lower andwill normally be applied on healthy skin. On the other hand consumer expo-sure is considered to last much longer than a shorter term treatment of a burn.ECHA (2011) http://apps.echa.europa.eu/registered/data/DISS-9d92ea78-89c7-2334-e044-00144f67d249/showdocument671a.html?treeUuid=DISS-9d92ea78-89c7-2334-e044-00144f67d249%2FDISS-9d92ea78-89c7-2334-e044-00144f67d249&uuid=AGGR-1d2a0e67-531a-489a-8bde-a3965db67638%2FDISS-9d92ea78-89c7-2334-e044-00144f67d2497
85
Better toxicity and exposure data are needed for improved risk assessment.However, based on existing data, indicating that the absorption via skin is loweven for damaged skin, it could be assumed that the health risk via this expo-sure route would be quite low.Risk following oral exposureAvailable toxicity data for oral exposure is mainly related to silver used in drugapplications. Based on human data (agyria) a limit in drinking water is set to0.01 mg/L corresponding to 0.02 mg/person/day. This value corresponds to2.5 % of an estimated human lifetime NOAEL of 10 g (corresponding to 0,4mg/person/day over a 70 years period).This value is much lower as compared to a DNEL value of 1.2 mg/kg bw/dfor bulk silver for systemic effects which has been derived by the registrant ofsilver in the REACH registration dossier on silver (ECHA web site April82011) , again indicating that nano-silver is more toxic than bulk silver.No quantitative data for consumer exposure to nanosilver have been identi-fied, and therefor no quantitative risk assessment can be performed.The oral route is not expected to be the most important route of exposure forconsumers, except from intake of nano or colloidal silver as food supplement,where high exposure could be expected. Consumer exposure via the oralroute could alsooccur, albeit at a relatively low level, if chewing on silver con-taining textiles or skin to mouth contact, from cosmetics containing nano-silver.However, relatively high exposures may occur from silver containing toothbrusches or toothpaste. If it is considered that toothpaste contain 0.002%nanosilver, and estimated that a person use 1 tube of toothpaste of 100g in 20days. As a worst case exposure estimate, assuming that 100% of the nanosilveris absorbed, will be 0.1 mg/person/day. This is 10 times higher than the limitfor drinking water in Denmark and 25% of the human NOAEL for silver.Existing knowledge baseNowacket al.(2011) refers to existing knowledge from the more than 50-yearuse of nanosilver for biocidal, algicidal and disinfectant uses and use of colloi-dal nanosilver for medical purposes. The authors conclude that the toxicity ofsilver is considered to be relatively low and that toxic effects on humans otherthan argyria are only observed at very high concentrations. Furthermore thaystate that a significant portion of the historical toxicological research on theeffects of silver on humans can be considered early examples of “nanotoxicol-ogy”.According to Nowacket al.(2011) data about effects on nonmammalian spe-cies for environmental risk assessment have until recently been obtainedmostly using dissolved silver, and nano-Ag is only studied as part of more re-cent research. The authors stress that it is questionable if data obtained usingionic silver should be used to derive threshold values in the environment assilver in natural waters typically is associated with the particulate or colloidalECHA (2011) http://apps.echa.europa.eu/registered/data/DISS-9d92ea78-89c7-2334-e044-00144f67d249/showdocument671a.html?treeUuid=DISS-9d92ea78-89c7-2334-e044-00144f67d249%2FDISS-9d92ea78-89c7-2334-e044-00144f67d249&uuid=AGGR-1d2a0e67-531a-489a-8bde-a3965db67638%2FDISS-9d92ea78-89c7-2334-e044-00144f67d2498
86
fraction and thus to some extent is naturally present as nanoparticles andmetal-sulfide clusters. Many aquatic species are much more sensitive to silvercompared to mammals and therefore the question whether nanosilver has adifferent toxicity compared to dissolved silver is extremely important.Based on the long historical record of relatively safe use and regulation of sil-ver including nanosilver, Nowacket al.(2011) argue that nanosilver is a mate-rial that does not fit the paradigm of "new" chemical with new and unknownrisks and therefore it is sufficient to apply the existing coherent risk assess-ment framework for other silver-containing materials.7.8Summary sheet for nano-Silver
Nanomaterial characteristicsNameCAS numberChemical compositionAppearanceSilver; NanoAg7740-22-4 (elemental Ag)AgWhite lustrous powder
Source:http://www.tradevv.com/
Manufacturing processes
Ultra-sonic precipitation, chemical vapour deposition, explodingwire synthesis. The size, shape, surface area, etc. can be modi-fied by adding various surface active agents and coatings tosyntheses involving silver salts.
Nanomaterial descriptionNanosilver (AgNP) is the nanoform of silver characterized by being spherical particles of sizesranging from 1-250 nm. AgNP is commercialized as powder, flakes, grains, ingots, etc., and is soldin suspension (in water, alcohol or surfactant) and as a dry powder. AgNP also available in prepa-rations (e.g. as a coating agent, in alloys, etc.) and in articles (electrodomestic appliances, in tex-tiles, in food packages, etc.). In its pure form AgNP will aggregate and hence nanosilver is oftensurface modified with for instance dextran, citrate, or PVP. Sometimes AgNP is also found to bedeposited on or used as a coating of a substrate such as plastic, silica or polymers, to give a de-sired adhesion, electrical conductivity, etc. In aqueous solutions AgNP forms dissolved free silverions in aqueous by dissolution and subsequent oxidation.ApplicationsThe use of AgNP is very diverse and include therapeutic applications (diet supplement), personalcare products, powdered colours, varnish, textile, paper, interior and exterior paints, printing col-ours, water and air-purification, polymer-based products and foils for antibacterial protection suchas washing machines, kitchenware and food storage. The AgNP concentrations used are unknownfor most applications. The scale of use of AgNP is unknown at this point in time, but expected toincrease rapidly as more and more consumer products with AgNP are entering the market.Human health risk profileIt has been shown that silver nanoparticles can be absorbed via especially the oral and inhala-
87
tional route, whereas intact skin is a barrier against absorption. However, it is unclear in whichform (as particles, free ions, silver ions or complexes) nanosilver is absorbed and distributed totarget organs. At least for uptake via the oral route it is likely that at least some of the uptake oc-curs as ions. It appears that smaller particles exhibit higher toxicity as compared to larger particles;and if silver is absorbed as particles then the surface area is relevant.Should silver uptake occur solely as ions, the database for silver could be applied to assess sys-temic silver nanoparticle toxicity. For that exercise, it would need to be considered whether andhow the dramatically increased surface area and possibly increased solubility of silver nanoparti-cles would need to be taken into account.Quite a few, mainlyin vitrostudies, have shown that the main mechanism of silver nanoparticletoxicity seems to be mediated by an increase in ROS production, stimulating inflammation andgenotoxic events and apoptosis or necrosis. The concentration of the administered nanoparticlesis able to influence the toxicity, specifically, at low levels of oxidative stress a protective response isinitiated which progresses to a damaging response with increasing particle concentration, andtherefore oxidant levels. It is thus relevant to consider that the toxicity of silver nanoparticles isthresholded.No data are identified about consumer exposure to nano-silver. The most important exposureroute for consumers is expected to be dermal exposure from textiles and cosmetics. However,from available data only minimal absorption is expected even on damaged skin, but furtherin vivostudies would result n more precise estimates.Consumer exposure via inhalation can occur from products used in spray form. Based on availabletoxicological data, it is recommended to avoid exposure via inhalation, because health effects likereduced lung function and toxic effect on the liver are considered to be the critical effects frominhalation.It is still unclear whether nano-silver is more toxic than bulk silver after oral exposure, especiallyafter long term exposure. The toxicity of silver after oral exposure is low, however after long termexposure silver can accumulate in the skin and different organs, which can result in a bluish-graycolouring of the skin (agyria) or deposition in the eyes (agyrosis). The effect on other organs afterlong term exposure is still unknown.For long term (life time) exposure a limit of 0.1 mg/L is set for silver in drinking water.Even though the oral exposure is not expected to be the main exposure route for consumers, expo-sure from tooth paste can occur and might be close to the exposure from drinking water. Highexposure could be expected from intake of food supplements containing nano- (colloidal) silver,which are available on the internet, but not approved for use in Europe.Environmental risk profileSilver is known to be an ecotoxic metal and test with silver nanoparticles (AgNP) do also revealvery low effect concentrations. Thus, for algae EC50-values as low as 4μg/Lhas been found andalso for crustaceans values far below 1 mg/L has been reported. This ranks AgNP as “AquaticChronic 1”. It is also important to note that at concentrations below 1 mg/L inhibition of nitrifyingbacteria can occur and thus the function of wastewater treatment plants may be affected by thepresence of AgNP. For ionic silver it is known that the speciation in aqueous media is determiningfor the bioavailability and toxicity. This is most likely also the case for elemental silver nanoparti-cles, but influence of speciation on uptake, depuration, and toxicity remain to be studied in thiscase.The environmental concentration resulting from the use of AgNP in consumer products are atpresent uncertain, even though a number of different estimates have been proposed. It has beendocumented that even though silver nanoparticles are incorporated in textiles, they can be re-leased upon washing. Concentrations in the low ng/l range have been proposed and even such lowconcentrations may under very precautious assumptions9constitute an environmental risk due tothe high toxicity of silver.It is debated today whether silver nanoparticles are in fact more toxic that their bulk counterpart,since effects in many cases can be ascribed to the ionic form of silver (Ag+). Some studies havedocumented a higher effect of AgNP, but it is the widespread and dispersive use of silver in con-sumer products that poses the greatest risk to the aquatic and terrestrial environment. Even ifAgNPs are “only” as toxic as larger silver particles, silver is still a metal of high environmental con-cern.9
Using the highest assessment factor of 1000 and that the bioavailability of silver isnot reduced due to complexation.
88
88.1
NanoclayGeneral characteristics
Nanoclays are minerals which have a high aspect ratio and with at least onedimension of the particle in the nanometer range. The most important factoris the aspect ratio of the clay particle. The clays having a platy structure and athickness of less than one nanometer are the clays of choice. Silica is thedominant constituent of clays followed by alumina. Depending on the chemi-cal composition and nanoparticle morphology, nanoclays can be organised inseveral classes including bentonite with montmorillonite as the principle clay10mineral consituent, kaolin and smectite. :Bentonite is the rock or ore that contains the clay mineral montmoril-lonite.Smectite is a clay mineral group characterized as an expanding claymineral which includes the clay minerals vermiculite, sauconite, sapo-nite, nontronite, hectorite and montmorillonite.Montmorillonite is a clay mineral species with a 2:1 expanding crystallattice whereas kaolinite is a 1:1 layer type.Cloisite� Na+ is a highly refined, untreated montmorillonite sold un-der the trademark of Cloisite� nanoclay by Southern Clay Products.
The terms Montmorillonite, Smectite and Bentonite are sometimes used in-terchangeably.Montmorillonite clays are the most commonly used nanoclays. Montmorillo-nite nano clays are unique clays having a platy structure with a unit thicknessof one nanometer or less but the surface dimension is generally 300 to morethan 600 nm. The aspect ratio is in the 1000:1 range (Sureshet al.,2010).Organically-modified nanoclays (organoclays) constitute a class of hybrid or-ganic-inorganic nanomaterials with potential uses in polymer nanocomposites,as rheological modifiers, gas absorbents and drug delivery carriers.8.2Manufacturing processes
Clays are mined and purified and then milled into the desired size.Because montmorillonite clay is hydrophilic, it is not compatible with mostpolymers and must be chemically modified to make its surface more hydro-phobic. The most widely used surface treatments are ammonium cations,which can be exchanged for existing cations already on the surface of the clay.A lot of research is going into surface modication of nanoclay (e.g. onuim ionmodification) in order to make nanoclay dispersed evenly in polymers which
10
From: http://www.nanoclay.com/faqs.asp
89
are generally organophilic (Nanocor 2010, Vejen-Henriksen et a. 2009, Briel2010).8.38.3.1UsesMain applications
The organically modified platelets have been proven to reinforce thermoplas-tics by enhancing flexural and tensile modulus while lowering CLTE (TheCoefficient of Linear Thermal Expansion). They are also effective at improv-ing gas barrier properties of thermoplastic systems. The surface char forma-tion and flame retardance of thermoplastic systems have also been improvedby incorporating the nanoclay particles into the structure. Other unique appli-cation areas have been shown to include improvement of the properties of in-jection molded pieces for the automotive industry, of flexible and rigid pack-aging such as films, bottles, trays, and blister packs, and also of electronicsplastics such as wire and cable coatings.Nanoclay has also been shown to improve the mechanical properties of ce-ment and result in higher compressive strength and tensile strength of cementmortars compared to plain cement with the same water-binder ratio (Morsy etal, 2010).The main application of nanoclay seems to be food packaging where they mayalso substitute materials based on petrochemical polymers. The platelet mor-phology of nanoclays forces gases to follow a complicated path through thepolymer, which slows down their transmission. The nano-layer structurethereby increases the path of diffusion that penetrating molecules of gases orother substances must take and significantly improves the polymer's barrierproperties. Examples of pacgaging materials include beer bottles (PEN 2010).Often the nanoclay is embedded in a polypropylene layer that is itself placedbetween external and internal polyethylene layers (Taylor 2008). No con-sumer products have been identified in the database to entail nanoclay on theDanish market.The nanoclay concentrations used in food packaging have not been identifiedand the scale of use is not known at this point in time.Other reported uses of nanoclays include the use as synergist flame retardantto substitute the halogen-containing flame retardants. Organoclays are notflame retardant agents as such, but they show synergistic effects with a broadrange of conventional flame retardants like aluminium hydroxide, magnesiumhydroxide as well as halogen- and phosphor-based flame retardants. By add-ing low concentrations of organoclay to thermoplastics the amount of conven-tional flame retardants can be reduced significantly (Pirngadi and Richter,2005).8.3.2Results from industry survey
The finest clay minerals (e.g the mineral smectite) are in reality nanomateri-als. Nanoclay is the main component of bentonite, which is defined as a claymaterial dominated by smectite. Bentonit is among other places extracted inDenmark (at Tåsinge). (Lindgreen, H., 2010).In Denmark nanoclay is e.g. utilised for production of leca (insulation materialbased on clay), drilling mud, and for tightening of drillings holes etc. Nano-
90
clay is known to be used in other countries as additive for lacquers etc. andprobably also plastic materials. From other countries also patents on the use ofnanoclay in cement exist. (Lindgreen, H. , 2010].Use of nanoclay in cement has also been tried in Denmark, but only as an ex-periment that now has been stopped (Damtoft, J.S., 2010].No information on import of nanoclay in finished products to Denmark iscould be obtained.The extraction of nanoclay in Denmark is anticipated to exceed 30.000tons/year (Dantonit, 2010).Information about use in food packaging was not obtained during the survey.8.4Eco-toxicological profile
No ecotoxicological studies of nanoclay have been identified as of 30 Novem-ber 2010. Since clay is a naturally occurring material for which environmentalorganisms have adapted throughout evolution, the inherent toxicity may canexpected to be low, however, issues related to small particle sizes may occur.8.5Toxicological profile
Today few studies are available and there is limited knowledge about the tox-icity of nanoclays and the chemical derivatives that may be generated duringproduction and processing of polymer nanoclay composites. However, ingeneral nanoclay is not considered to pose a major health risk although a pos-sible content of crystalline quartz may constitute a risk. Furthermore func-tionalised nanoclays containing quaternary ammonium or phosphonium func-tional groups on the surface are described as potentially problematic, as am-monium and phosphonium ions in their pure form can cause asthma symp-toms (NFA, 2010).8.5.1
Liet al.(2010) investigated the acute toxicity of nanoclay fed to Sprague-Dawley rats and found LD50 to be greater than 5700 mg/kg bw for both maleand female rats.Warheitet al.(2010) compared the pulmonary and extrapulmonary systemicimpacts in rats of intratracheally instilled Sepiolite nanoclay samples withquartz or ultrafine titanium dioxide particle-types at doses of 1 mg/kg or 5mg/kg. Dedicated groups were evaluated by bronchoalveolar lavage, lung cellproliferation, macrophage functional assays and full body histopathology atselected times postexposure (pe). Bronchoalveolar lavage results demon-strated that quartz particles produced persistent, dose-dependent lung in-flammatory responses measured from 24 h through 3 months pe. Exposuresor Sepiolite samples produced transient neutrophilic responses at 24-h pe;however, unlike the other particle-types, Sepiolite exposures producedmacrophage-agglomerates or multinucleate giant cells at 1 week, 5 weeks and3 months pe. Lung parenchymal cell proliferation rates were increased in ratsexposed to quartz but not Sepiolite. Histopathological evaluation of lung tis-sues revealed that pulmonary exposures to Sepiolite nanoclay or quartz sam-ples produced inflammation in centriacinar regions at 24 hours pe but the ef-
91
fects decreased in severity over time for Sepiolite and increased for quartz-exposed rats. The quartz-induced lesions were progressive. In the Sepiolitenanoclay group, the finding of multinucleated giant cell accumulation associ-ated with minor collagen deposition in acinar regions was rarely observed. Fullbody histopathology studies were conducted at 24 h and 3 months post parti-cle exposures. Histopathological evaluations revealed minor particle accumu-lations in some mediastinal or thoracic lymph nodes. No extrapulmonary tar-get organ effects were observed in any of the particle-exposed groups at 3months postexposure.8.5.2
Lordanet al.(2011) evaluated the cytotoxic effects induced by unmodified(Cloisite Na+ �) and organically modified (Cloisite 93A�) nanoclays in thehuman hepatic HepG2 cell line. Following 24h exposure the nanoclays sig-nificantly decreased cell viability. Cloisite Na+ induced intracellular reactiveoxygen species (ROS) formation which coincided with increased cell mem-brane damage, whilst ROS generation did not play a role in Cloisite 93A-induced cell death. Neither of the nanoclays induced caspase-3/7 activation.Moreover, in the cell culture medium the nanoclays aggregated differently andthis appeared to have an effect on their mechanisms of toxicity. Based on re-sults from the study the authors concluded that nanoclays are highly cytotoxicand as a result pose a possible risk to human health.Liet al.(2010) evaluated thein vitrocytotoxicity and genotoxicity of exfoli-ated silicate nanoclay using the Comet assay in Chinese Hamster Ovary(CHO) cells, a micronucleus test and the Salmonella gene mutation assay onstrain TA98, TA100, TA102, TA1535 and TA1537. The Comet assayshowed no DNA damage after 24 h of incubation with NSP of 1000g/mL,no significant micronucleus induction was observed in the CHO cells at theconcentrations tested, and no of mutations were found in the five Salmonelleastrains. Furthermore, cytotoxicity of the same material was assayed, showing alow cytotoxicity on CHO cells below 1000 microg/mL after 12 h incubationperiod and a dose-dependent effect after 24 h incubation.Sharmaet al.(2010) investigated the potential of filtered and unfiltered watersuspensions of the natural clay mineral montmorillonite (Cloisite� Na+) andan organo-modified montmorillonite (Cloisite�30B) in the Salmo-nella/microsome assay at concentrations up to 141g/mlof the crude clay,using the tester strains TA98 and TA100. Both clays did not induce muta-tions in this assay. Filtered and unfiltered Cloisite�Na+ suspensions in cul-ture medium did not induce DNA strand-breaks in Caco-2 cells after 24 h ofexposure, as tested in the alkaline comet assay. However, both the filtered andthe unfiltered samples of Cloisite�30B induced DNA strand-breaks in a con-centration-dependent manner and the two highest test concentrations pro-duced statistically significantly different results from those seen with controlsamples. When tested in the same concentration range as used in the cometassay, none of the clays produced ROS in a cell-free test system (the DCFH-DA assay). Inductively coupled plasma mass-spectrometry indicated that clayparticles were absent in the filtered samples, which was independently con-firmed by dynamic light-scattering measurements. Detection and identifica-tion of free quaternary ammonium modifier in the filtered sample was carriedout by HPLC-Q-TOF/MS and revealed a total concentration of a mixture ofquaternary ammonium analogues of 1.57 mu g/ml. These findings suggestthat the genotoxicity of organo-modified montmorillonite was caused by theorgano-modifier. The detected organo-modifier mixture was synthesized and
92
comet-assay results showed that the genotoxic potency of this synthesized or-gano-modifier was in the same order of magnitude at equimolar concentra-tions of organo-modifier in filtrated Cloisite (R) 30B suspensions, and couldtherefore at least partly explain the genotoxic effect of Cloisite (R) 30B.8.5.3Summary
The acute toxicity of nanoclay is low based on the results of a rat study. Tran-sient inflammatory reactions were observed in rats after intratracheal instilla-tion of Sepiolite nanoclay but no extrapulmonary target organ effects wereobserved following the exposure.No consistent picture regarding cytotoxicity and genotoxicity of nanoclayscan be drawn based on the available studies.8.6Exposure scenarios
There is so far not much information about exposure to nanoclays. The use ofnanoclay embedded in polymers is in general not considered to give rise to arisk of exposure neither to humans norin the environment during the usephase of the products although some experiments indicate that nanoclay canbe released from polymer nanocomposite (PNC) film (NFA, 2010). Nano-clays are typically used in concentrations up to 5% in polymer materials (En-vironmental Project No. 1206, 2007).When used for other applications e.g. insulation materials or in conncetionwith other construction work a potential for dermal and inhalational exposureshould be considered.After disposal of the products, nanoclay may be released, but since nothing isknown today about release of nanomaterials during waste management opera-tions (e.g. recycling, landfilling, or incineration), exposure estimations cannotbe made.8.78.7.1Risk profileEnvironment
No ecotoxicological studies of nanoclay have been identified as of 30 Novem-ber 2010. Since clay is a naturally occurring material for which environmentalorganisms have adapted throughout evolution, the inherent risk may be ex-pected to be low, however, issues related to small particle sizes may occur.8.7.2Human health
Nanoclay has low acute toxicity in animal experiments and is normally notconsidered to constitute a significant health hazard except for the possible ef-fects on the lungs from inhaling dust. For assessing risk in relation to con-struction applications and dermal and inhalational exposure there are no spe-cific data available related to the nanoform of the clay, so it is uncertain towhich extent nanoclay have another toxicological profile compared to clayparticles in general. A possible content of crytalline quartz (up to 0.5 %) mayadd to the risk from inhalation of the material. Likewise a possible content ofquaternary ammonium or phosphonium functional groups on the surface may
93
potentially be problematic, as ammonium and phosphonium ions in their pureform can cause asthma symptoms (NFA, 2010).In general the risk in relation to nanoclay is related to the inhalation of mineraldust which should always be minimised independent of the the size and formof the particles.8.8Summary sheet for nanoclay
Nanomaterial characteristicsNameCAS numberChemical compositionNano-clayNot applicableSilica/alumina containingmineralFine powderSource:http://www.nanocor.com
AppearanceManufacturing processesNanomaterial description
Clay is mined and purified and then milled into the desired size.
As it is the case for clay, nano-clay consists of finely grained alumina silicate minerals like mont-morillonite, which is a 2-to-1 layered smectite clay mineral. Individual platelets are just about onenanometer thick, but surface dimension are generally 300 to more than 600 nm. Natural mont-morillonite is hydrophilic and a lot of research is going into surface modication of nanoclay (e.g.onuim ion modification) in order to make nanoclay dispersed evenly in polymers which are gener-ally organophilic.ApplicationsMain application of nanoclay seems to be food packaging e.g. in beer bottles. Often the nanoclayis embedded in a polypropylene layer that is itself placed between external and internal polyethyl-ene layers. The nanocaly concentrations used in food packaging are unknown and so is the scale ofuse is unknown at this point in time.Human health risk profileNanoclay has low acute toxicity in animal experiments and is normally not considered to constitutea significant health hazard except for the possible effects on the lungs from inhaling dust. A possi-ble content of crytalline quartz (up to 0.5 %) may add to the risk from inhalation of the material.Likewise a possible content of quaternary ammonium or phosphonium functional groups on thesurface has been discussed as potentially problematic as ammonium and phosphonium ions intheir pure form can cause asthma symptoms. No dose-response relationships are established.Environmental risk profileNo ecotoxicological studies of nano-clay have been identified as of 30 November 2010. Since clayis a naturally occurring material for which environmental organisms have adapted throughoutevolution, the inherent toxicity may can expected to be low, however, issues related to small parti-cle sizes may occur.
94
9 Silicium dioxide SiO29.1General characteristics
Silica or silicon dioxide (SiO2) is an abundant mineral commonly found innature in the form of sand or quartz as well as in cell walls of diatoms. SiO2has a number of distinct crystalline forms (rhombohedral, hexagonal, ortho-rhombic) in addition to amorphous forms e.g.α-quartz, β-quartz.AmorphousnanoSilica particles can be produced in all sizes e.g. one manufacturer hasnanosilica particles available in the size of 9, 15, 30 and 55 nm with a specific2surface area of 300, 200, 100 and 50 m /g (Bayercoatings 2010). The shapeof nanosilica is normally spherical, but other forms exsist as well such as forinstance springs and nanosilica can also vary in regard to their porousity.Nanosilica can furthermore be functionalized with instance organogroups aswell as other metals (mknano 2010).9.2Manufacturing processes
Several methods can be used produce nanoparticles. NanoSilica can be pro-duced via the sol-gel method where silica monomers are allowed to condenseto colloidal particles, which again can form aggregates and age. Anothermethod is high-temperature hydrolysis in a hydrogen oxygen flame. The termpyrogenic silica refers to highly dispersed silicas formed from the gas phase athigh temperature. The vapor-liquid-solid method can be used to produce sil-ica nanospring (Lidong et al. 2006). Silica nanoparticles are often used informulation or as components of other nanoparticles. as e.g. a surface silaniza-tion may result in less cytotoxic nanoparticles suited for bio-applications.9.39.3.1UsesMain applications
Silica nanoparticles have numerous applications such as ingredients in ce-ment, paints, solid lubricants, shampoos, cosmetics, face creams and food(spices) (Wiesner 2005, Environmental Project No. 1206, 2007, Winteret al.2010, PEN 2010). Bioelectrocemistry, curing, oil recovering applications andin formulation of other particles are furthermore potential application (Sunetal.2009 Makimuraet al.2010). Organo-silica has also been reported to beused as scratch resistant coatings (Boxallet al.2008). No products were iden-tified using silica nanoparticles on the Danish marked, but a total of 14 prod-ucts were identified that use the non-oxidized form of silica, namely silicon(Si) . Of these products nine products were in the category Health and Fit-ness.Very little information is available about the SiO2concentrations used in thevarious products. Concentrations of 0.1% and 15% as been reported forpaints, whereas organo-silica is used in concentrations of 10% in scratch resis-tant coatings are reported. Very little is furthermore known about the scale ofuse, but one Danish company has been reported to handle 1-10 ton silica peryear (Environmental Project No. 1206, 2007, Boxallet al.2008).
95
9.3.2
Results from industry survey
Microsilica is a residual from manufacturing of ferrosilicium with a size of 1-10 nm. Microsilica may be used in concrete as well as in paint etc. The sur-vey on the use of microsilica has been focused on concrete assumed to be adominant field of application-In concrete microsilica has been used since the 1980'es. Use of microsilicaprevents alkali-silica reactions that may cause concrete to be torn apart. Mi-crosilica furthermore has a puzzulane effect and may increase the tightnessand thereby the strength of concrete. Previously microsilica was widely usedfor manufacturing of concrete in Denmark. Today the use has been signifi-cantly reduced, primarily because of strong price increases due to heavy de-mand from the Middle East. (Eriksen, 2010). According to EnvironmentalProject No. 1206 (2007), the commercially available silica nanoparticles (Mi-crosilica) with a grain size of 100-200 nm are used in the production of con-crete. In dry form the used silica nanoparticles are aggregated with an aggre-gate size above 100 nm. When dispersed in water the aggregates break up intoprimary particles smaller than 100 nm.The concent of microsilica used in concrete will correspond to 4-6 % (weight-based) of the amount of cement (Eriksen, 2010).The present consumption of microsilica in concrete in Denmark may beroughly estimated to at least 3. - 4.000 tonnes/year (Jensen, 2010).9.4Eco-toxicological profile
For the following overview of the ecotoxicological profile of nano-scale silica(SiO2) it should be stressed that only a few ecotoxicological studies exist andonly a part of these are aimed at the base-set organisms (fish, crustacean andalgae) required for doing effects assessment according to REACH. Evenfewer studies report the results in terms of the endpoints and values listed inInformation Requirements of the REACH (e.g., LC50, EC50, NOEC,LOEC) (ECHA, 2008).For zebrafish embryos (Danio rerio) significant incidences of jaw malforma-tions was noticed at the concentration of 250 mg/L, but other effects were notreported as result of the SiO2/AlO2nanoparticles (Harperet al.2008). Silicananowires of 200 nm and 50 nm) with aspect ratios (i.e. ratio between lengthand diameter) greater than 1 cause has been found to cause zebrafish embryodeformities, but particles with aspect ratio of 1 did not exhibit any toxic orteratogenic activities at the same concentrations (Nelsonet al.2010).A study by Fentet al.2010 assessed the uptake and embryo toxicity of fluo-rescent silica nanoparticles in zebrafish (Danio rerio). Two different size fluo-rescent silica nanoparticles (~200nm and~60nm)were used. Zebrafish em-bryos were exposed to concentrations of 0.0025-200 mg/L. No significantchanges were observed in the survival time, hatching time, the morphology ofembryos or larvae or development of zebrafish eggs.For the freshwater crustacean Daphnia magna exposed it was found that SiO2concentrations of 10 mg/L caused 70% mortality (Adamset al.2006).Growth inhibition tests using the algae Chlorella kessleri showed that the sizeof cells increased as a result of exposure with the 5 nm SiO2particles and that
96
these had a greater effect than the 26 and 78 nm particles (Fujiwaraet al.,2008). Growth rate inhibition of the green algae Pseudokirchneriella subcapi-tata (72 h incubation) was observed after exposure to 12.5 nm and 27 nm sil-ica NPs by van Hoeckeet al.(2008). EC10, 72h were found to be 10.9 � 4.4mg/L and 15.0 � 4.3 mg/L, and NOEC and LOEC were observed to be 4.6and 10.0 mg/L for 12.5nm and 27 nm SiO2NPs, respectively. Taking thespecific surface area of the silica particles into account, van Hoeckeet al.(2008) also expresse EC10-values as EC10, 72h in units of m2/l yielding val-ues of 2.6 � 1.0 and 2.0 � 0.6 m2/L 12.5nm and 27 nm SiO2NPs respec-tively. The toxicity of silica nanoparticles (10-20 nm) and the bulk particles(5-10μm) towards the green algae Scenedesmus obliquus revealed EC20 val-ues of 388.1 mg/L (72 h) and 216.5 mg/L (96 h) (Weiet al.2010).No studies on the bioaccumulation of SiO2have been found as of 22 October2010. By definition metal oxide nanoparticles are not degradable and speci-ation of SiO2(e.g. dissociation or complexation) is not likely to occur inaquatic media under normal conditions. Therefore, SiO2particles can be re-garded as persistent in the sense that the particles will remain as SiO2-particles, however the sizes of the particles may change due to agglomerationand aggregation in aquatic media.9.5Toxicological profile
Most of the available toxicological research into bulk silica has focussed oncoarse or fine crystalline silica particles of 0.5 to 10m.Crystalline silica inthe form of quartz is an IARC classified human carcinogen and is involved inthe pathogenesis of silicosis. However, the mechanisms of crystalline silicatoxicity at the cellular or molecular levels are still unclear. It is also not knownif any single mechanism underlies the observed effects although severe in-flammation appears to be a common initiating step. The role of reactive oxy-gen species in the inflammatory, fibrogenic and carcinogenic activity of quartzis well established. Oxidative membrane and DNA damage are considered themost important mechanisms involved in the health effects of microsized crys-talline silica (Napierskaet al.,2010). A large body of experimental work hasshown that particle surface reactivity and the form of silica seem to govern thehazardous nature of crystalline silica and that cytotoxicity appears to be di-rectly related to the form of the particles and the extent of the exposed sur-face.Amorphous silica and the naturally occurring amorphous silica such as diato-maceous earth are in general considered less harmful than its crystalline coun-terpart. With regard to the cytotoxic activity of different forms of amorphoussilica, it has been shown not to depend on a crystalline silica component butrather on the surface charges and morphologic features of particles (Napier-skaet al.,2010).For the silica nanoparticles (SNPs) the origin/synthesis of the particles playsan important role in determining the physico-chemical properties and conse-quently their potential interactions with biological systems. Relevant parame-ters are surface area, surface morphology, surface energy, dissolution layerproperties, adsorption and aggregation properties (Napierskaet al.,2010).Nanosilica occurs mainly in the amorphous form, and the potential hazardcannot be simply related to studies of micron-sized crystalline materials.
97
9.5.1
ADME studies
No studies investigating the absorption from the site of exposure into theblood have been identified. Onein vivostudy investigating the impact of sizeon tissue distribution and elimination of SNPs has been identified. In thisstudy nanosilica (50, 100, and 200 nm diameter) injected intravenously intomice was observed in macrophages of the liver and spleen for four weeks aftera single injection. The study also showed that the smaller particles werecleared via urine and bile more rapidly than larger particles (Choet al.,2009).Kumaret al.(2010) studied biodistribution of multimodal organically modi-fied SNPs intravenously administered to nude mice and found a greater ac-cumulation of nanoparticles in liver, spleen, and stomach than in kidney,heart, and lungs. Almost 100% of the injected nanoparticles were effectivelycleared out of the animals over a period of 15 days via the hepatobiliary excre-tion. No signs of organs toxicity were observed.In anin vitrostudy in human mesenchymal stem and 3T3-L1 cells the effectof surface charge on cellular uptake of mesoporous nanosilica (porous silicateswith very big surface areas) was evaluated. The results showed that the uptakecould be regulated by a threshold of positive surface charge and also implythat the modulation of surface charge is cell specific (Chunget al.,2007).9.5.2Short term toxicity
InhalationSixin vivostudies, one involving inhalation and five involving intratrachealinstallation of a single dose of different forms of silica in rats and mice havebeen identified.Sayeset al.(2010) exposed rats by inhalation to freshly generated, aerosolisedamorphous silica NP for 1 or 3-days. Neither exposure period produced anysignificant pulmonary inflammatory, or adverse lung histopathological effectsin rats exposed to very high particle numbers corresponding to a range of3mass concentrations (1.8 or 86 mg/m ). It was also concluded that there wasno genotoxic effects from the exposure.In a study with intratracheal instillation of different sizes of nanoquartz andmined quartz particles in rats the theory that nanoparticles are more toxic thanfine-sized particles of similar chemistry was contradicted. Well-characterizedsamples were tested for surface activity and hemolytic potential. In addition,groups of rats were instilled with either doses of 1 or 5 mg/kg of carbonyl iron(CI) or various alpha-quartz particle types in phosphate-buffered saline solu-tion and subsequently assessed using bronchoalveolar lavage fluid biomarkers,cell proliferation, and histopathological evaluation of lung tissue at 24 h, 1week, 1 month, and 3 months postexposure. Exposures to the various alpha-quartz particles produced differential degrees of pulmonary inflammation andcytotoxicity, which were not always consistent with particle size but correlatedwith surface activity, particularly hemolytic potential. The results demonstratethat the pulmonary toxicities of alpha-quartz particles appear to correlate bet-ter with surface activity than particle size and surface area (Warheitet al.,2005).Another study with intratracheal instillation of colloidal silica in rats revealedthat the effect of fibrogenesis of nanosized SiO2might be milder than that ofmicrosized SiO2in rats, potentially resulting from nanoparticles tending to be
98
diffused and easily translocated due to their ultrafine particle size compared tomicrosized particles (Chenet al.,2004).Kaewamatawonget al.(2006) compared acute pulmonary toxicity from expo-sure to ultrafine colloidal silica particles (UFCSs) and fine colloidal silica par-ticles (FCSs) in mice intratracheally instilled with 3 mg of 14 nm UFCSs and230 nm FCSs revealed for both sizes bronchiolar degeneration, necrosis, neu-trophilic inflammation, alveolar type II cell swelling and alveolar macrophageaccumulation. UFCs induced extensive alveolar hemorrhage, a more severebronchiolar epithelial cell necrosis and neutrophil influx in alveoli comparedto FCSs. Electron microscopy demonstrated UFCSs and FCSs on bronchio-lar and alveolar wall surface as well as in the cytoplasm of alveolar epithelialcells, alveolar macrophages and neutrophils. Type I alveolar epithelial cell ero-sion with basement membrane damage in UFCSs treated animals was moresevere than those in FCSs-treated animals. These findings suggest thatUFCSs have greater ability to induce lung inflammation and tissue damagesthan FCSs.Acute and subacute lung toxicity of low dose of UFCSs was further studied inmice intratracheally instilled with 0, 0.3, 3, 10, 30 or 100gof UFCSs (Kae-wamatawonget al.,2006). Cellular and biochemical parameters in bronchoal-veolar lavage fluid (BALF), histological alteration and the body weight weredetermined at 3 days after instillation and in addition the time response wasinvestigated. UFCSs induced moderate pulmonary inflammation and tran-sient injury on BALF indices. UFCSs-treated animals showed a significantincrease of the apoptotic index in lung parenchyma at all observation times.Findings from the study suggest that instillation of a small dose of UFCSscauses transient acute moderate lung inflammation and tissue damage. Oxida-tive stress and apoptosis may underlie the lung tissue injury induction.Choet al.(2007) studied the pulmonary effects and inflammatory mecha-nisms of ultrafine amorphous silica particles (UFASs) intratracheally adminis-tered to A/J mice at doses of 0, 2, 10 and 50mg/kg (n=5 per group). Bron-choalveolar lavage fluid analysis, histopathological examination, quantitativereal-time PCR and immunohistochemistry of the lung tissues were assessed.UFASs significantly increased the lung weights and total BAL cells followingexposures. The histopathological examination revealed that UFASs inducedtransient but severe lung inflammation, with neutrophils, at an early stage andchronic granulomatous inflammation at the later stage. Cytokines (IL-1beta,IL-6, IL-8 and TNF-alpha) and chemokines (MCP-1 and MIP-2) were iden-tified as playing important roles in the inflammation induced by the intratra-cheal instillation of UFASs.DermalNoin vivodermal studies investigating short term toxicity have been identi-fied.In a study designed to determine whether nanosilica has the potential to causeacute cutaneous toxicity, using cultured HaCaT keratinocytes (CHK) Parketal.(2010) evaluated cytotoxicity of two nanosilicas of particle size 7 and 10-20 nm. HaCaT cell viabilities were determined after exposure to the twonanosilicas at different concentrations for 48 h. Both particle sizes dose-dependently reduced cell viabilities (P < 0.001). No differences were foundfor the two particle sizes.
99
OralNo short term oral studies have been identified.Intravenous, intraperitoneal, subcutaneousHudsonet al.(2008) studied the biocompatibility of mesoporous silicates andfound that when particles were injected subcutaneously in rats, the amount ofresidual material decreased progressively over 3 months, with good biocom-patibility on histology at all time points. In contrast, intra-peritoneal and intra-venous injections in mice resulted in death or euthanasia. No toxicity was seenwith subcutaneous injection of the same particles in mice. Microscopic analy-sis of the lung tissue of the mice indicated that death may be due to thrombo-sis. Although local tissue reaction to mesoporous silicates was benign, theycaused severe systemic toxicity.Nishimoriet al.(2009) investigated the relationship between particle size andtoxicity using silica particles with diameters of 70, 300 and 1000 nm (SP70,SP300, and SP1000) as a model material. Silica particles were administeredintravenously to mice and to evaluate acute toxicity, histological analysis wasperformed for liver, spleen, kidney and lung. SP70-induced liver injury at 30mg/kg body weight, while SP300 or 1000 had no effect even at 100 mg/kg.Administration of SP70 dose-dependently increased serum markers of liverinjury, serum aminotransferase and inflammatory cytokines. Repeated ad-ministration of SP70 twice a week for 4 weeks, even at 10mg/kg, caused he-patic fibrosis. These findings indicate that nano-size materials may be hepato-toxic.Park and Park (2009) investigated oxidative stress and proinflammatory re-sponses induced by silica nanoparticlesin vivoandin vitro.A single treatmentof SNPs (50 mg/kg) administered intravenously to mice led to the activationof peritoneal macrophages, increased cytokines, and increased level of nitricoxide released from the peritoneal macrophages. mRNA expressions of in-flammation-related genes were also elevated in the cultured peritoneal macro-phages harvested from the treated mice. Viability of splenocytes from themice treated with silica nanoparticles was significantly decreased in the higherdose-treated groups (100 mg/kg, 200 mg/kg i.p.). However, cell proliferationwithout cytotoxicity was shown in the group treated with the low dose of 50mg/kg i.p.To elucidate the pro-inflammatory mechanism of silica nanoparticlesin vivo,in vitrostudy using RAW 264.7 cell line which is derived from mouse perito-neal macrophage was carried out. Treatment of silica nanoparticles to the cul-tured RAW264.7 cells led to the reactive oxygen species (ROS) generationwith a decreased intracellular GSH. In accordance with ROS generation, silicananoparticles increased the level of nitric oxide released from the culturedmacrophage cell line. These results suggested that silica nanoparticles gener-ate ROS and the generated ROS may trigger the pro-inflammatory responsesbothin vivoandin vitro(Park and Park, 2009).9.5.3Irritation and corrosion
Parket al.(2010) studied dermal irritation in a Draize test according to KoreaFood and drug Administration guideline. Nanosilicas (7 and 10-20 nm) wereapplied to the shaved back of rabbits. No erythema or edema was observedafter exposure for 24 or 72 hours and the results suggest that nanosilica doesnot induce acute cutaneous irritation.
100
In the same study Parket al.(2010) also carried out a skin irritation study us-ing a human skin equivalent model, EpiDerm. Models were treated with 500g/mlof either nanosilica type for 5 and 18 h. Use of the model revealed noirritation potential at 500g/mlnanosilica.9.5.4Skin and respiratory sensitization (in vitro
andin vivo
)
No studies addressing sensitisation in relation to nanosilica have been identi-fied.9.5.5Repeated dose toxicity, short term, sub-chronic and long term
Repeated dose: inhalationTwo studies have investigated the pulmonary responses in rats after up to 4weeks inhalation of Ludox colloidal silica. Warheitet al.(1991) exposed CDrats (nose-only) for 2 or 4 weeks at concentrations of 0, 10, 50, and 1503mg/m Ludox (dried SiO2). Additional groups of rats exposed for 4 weekswere given a 3-month recovery period. Following exposure and/or recovery,fluids and cells were recovered from the lungs by bronchoalveolar lavage(BAL) and measured for cellular and biochemical parameters. Results showed3that exposures to 150 mg/m Ludox for 2 or 4 weeks produced pulmonaryinflammation along with increases in BAL protein, LDH, and alkaline phos-phatase values (p less than 0.05) and reduced macrophage phagocytosis.Autoradiographic studies demonstrated that the labelling indices of terminalbronchiolar and lung parenchymal cells were generally increased in the 50 and3150 mg/m groups after 2 and 4 weeks of exposure but, with one exception,returned to normal levels following a 3-month postexposure period. No sig-nificant alterations in any measured parameters were detected in rats exposed3to 10 mg/m Ludox at any time postexposure. The no-observable-effect level3(NOEL) was determined at 10 mg/m which was consistent with results ob-tained by conventional toxicology methods.Lee and Kelly (1992) exposed rats for 4 weeks at concentrations of 0, 10, 50,3and 150 mg/m to Ludox colloidal silica. Rats were killed after 4 weeks of ex-posure and 10 days or 3 months after exposure. No effects were seen at thelowest concentration. After 4 weeks exposure, lung weights were increasedsignificantly in the two highest dose groups but they were similar to the con-trols after 3 months. A dose dependent alveolar macrophage response, poly-morphonuclear leukocytic infiltration, and Type II pneumocyte hyperplasia inalveolar duct regions was reported. Lung-deposited nanosilica cleared rapidlyfrom the lungs with half-times of approximately 40 and 50 days for the 503and 150 mg/m groups, respectively. The lungs did not show fibrotic scar tis-sue formation or alveolar bronchiolarization.Chenet al.(2008) investigated age-related differences in pulmonary and car-diovascular responses to SiO2nanoparticle in a study with young, adult, andold rats exposed toair containing aerosol of manufactured SiO2nanoparticles3(24.1 mg/m ; 40 min/day) for four weeks. Inhalation of SiO2nanoparticlesunder identical conditions caused pulmonary and cardiovascular alterations inold rats, and less change in young and adult rats, including pulmonary in-flammation, myocardial ischemic damage, atrio-ventricular blockage, and in-crease in fibrinogen concentration and blood viscosity. Old individuals weremore sensitive to nanoparticle exposure than the young and adult rats. Therisk of causing pulmonary damages was: old > young > adult and the risk ofcardiovascular disorder was observed only in old age. The results suggest that
101
different ages may require different biomarkers for identifying pulmonary tox-icity during inhalation of nanoparticles.Repeated dose: dermalNo dermalin vivostudies with exposure to nanosilica have been identified.Repeated dose: oralSoet al.(2008) studied the effect of micro/nanoparticle silica fed to Balb/cand C57BL/6J mice for 10 weeks. The size of the nano and micron sized silicawere approximately 30 nm and approximately 30 microm, respectively.Bloodwas tested biochemically and hematologically. There was no difference be-tween the groups in the tested items except ALT (Alanine Aminotransferase).The nano sized silica particle dieted group showed higher value of ALT thannormal and micron sized silica dieted groups. H&E staining of the liver of thenano sized particle dieted group indicated some fatty liver pattern while thecontents of Si in the livers of the groups were almost the same. From the re-sults, it was suggested that the nanosized silica particle had a toxic effect onthe liver even though there was no significant difference in the health of micefed amount of 140 g silica/kg mouse.9.5.6Mutagenicity/genotoxicity
Lisonet al.(2009) investigated the genotoxic potential of SNP with thein vi-trocytochalasin-B micronucleus (CBMN) test. This test was selected becauseit allows the detection of two types of chromosomal damage, i.e. clastogenicevents or chromosome breaks and aneugenic events or chromosome loss. TheCBMN assay was applied in A549 cells treated with different concentrationsof 3 types of Stöber silica nanoparticles (16, 60 and 101 nm SNPs). A statisti-cally significant increase of micronuclei in binucleated cells was detected aftertreatment but only with the smallest SNPs, indicative of a size dependentgenotoxic effect. However, results showed no dose-dependent effect.Barneset al.(2008) reported no significant genotoxicity of commercial colloi-dal and laboratory-synthesized silica nanoparticles tested using the single cellgel electrophoresis or Comet assay. Comet assays were performed on 3T3-L1fibroblasts with 3, 6, and 24 h incubations and 4 or 40μg/mlof silicananoparticles. Results were independently validated in two separate laborato-ries.In a review document discussing adaptations of thein vitroMN (micronu-cleus) assay for the genotioxicity assessment of nanomaterials, Gonzaleset al.(2011) report on genotoxic effects of SiO2nanoparticles investigated in twostudies. Wanget al.(2007 as cited by Gonzales) demonstrated that crystalline7.21 nm SiO2nanoparticles induce MN. In contrast, no statistically significantinduction of MN was found by amorphous SiO2nanoparticles of 16, 60 and104 nm. In this study, MN frequencies were expressed in function of massdose, number of particles and surface area and this when taking into accountboth the nominal and cellular dose. They showed that there was a statisticallysignificant positive correlation between the fold increase of MN and the parti-cle number or surface area, considering both nominal and cellular dose. Nosignificant correlation was found between the fold increase of MN and massdose.Parket al.(2010) investigated the potential of four well-characterized amor-phous silica nanoparticles to induce chromosomal aberrations and gene muta-tions using the micronucleus and the plasmid lacZ gene mutation assay. The
102
80 (34) nm silica nanoparticles induced chromosomal aberrations in the mi-cronucleus assay using 3T3-L1 mouse fibroblasts and the 30 (34) and 80(34) nm silica nanoparticles induced gene mutations in mouse embryonic fi-broblasts carrying the lacZ reporter gene. TEM imaging demonstrated thatthe majority of nanoparticles were localized in vacuoles and not in the nucleusof 3T3-L1 cells, indicating that the observed DNA damage was most likely aresult of indirect mechanisms. The authors concluded that further studies areneeded to reveal these mechanisms and to determine the biological relevanceof the effects of these particular silica nanoparticlesin vivo.9.5.7Carcinogenicity
No carcinogenicity studies involving SNPs have been identified.9.5.8Reproductive toxicity, developmental toxicity and teratogenicity
No reproductive toxicity, developmental toxicity and teratogenicity studiesinvolving SNPs have been identified.9.5.9
Most studies carried out for mainly amorphous NSPs arein vitrostudies indifferent cell types. As summarised by Napierskaet al.(2019), most of thesein vitrostudies involving different SNPs documented the cytotoxic effects ofthese nanomaterials. The determinants of the observed cytotoxicity seem tobe complex and vary with the particles used and cell type tested. Unfortu-nately, for many published studies, adequate material characterization is stillmissing.Singhet al.(2009) also summarises results fromin vitrostudies: Silica hasbeen shown to induce inflammatory and oxidative stress responsesin vitroand alsoin vivo,but cytotoxicity is largely only observed at high concentra-tions. Additionally, silica nanoparticles have been shown to enter the cell nu-cleus where they could potentially bind to the DNA phosphate backbone.Since the silica nanoparticles can result in increased ROS (reactive oxygenspecies) levels and given that the hydroxyl radical is a highly reactive moleculethe generation of OH close to the DNA could readily lead to the induction ofDNA strand breaks and oxidised bases which could have important implica-tions in the development of cancer.Singhet al.(2009) further summarises: Silica nanoparticles also have an im-pact on nuclear integrity by forming intranuclear protein aggregates that canlead to inhibition of replication, transcription, and cell proliferation. Addition-ally, they have been shown to reduce replication activity down to 67% and60% after 6 and 24 h respectively and transcriptional activity down to 82%after 4 h, thus resulting in decreased cell proliferation after 24 h (88%) and 48h (65%). This study underscores the importance of nanoparticles sizing sinceparticles >200 nm fail to penetrate the nucleus, do not alter nuclear structureand function, and also do not interfere with gene expression.There seems to be conflicting results as regards the genotoxicity of SNPs. Onthe one hand, there is limited evidence to suggest silica nanoparticles aregenotoxic and some recent studies utilizing the comet assay have demon-strated that silica nanoparticles ranging in size from 20 to 400 nm do not exertsignificant genotoxicity (Singhet al.,2009). In contrast, one investigationbased on the micronucleus assay found that these nanoparticles do indeed in-
103
duce chromosomal damage. Thus, to get a clear indication of genotoxic po-tential a battery of standardised tests that quantify different types of geneticaberrations are required to cover all potential forms of DNA damage that maybe induced following exposure to nanoparticles.9.5.10 SummaryFor nano-silica particles there are relatively few studies of the toxicity availablecompared to what is available for titanium dioxide. Most studies arein vitrostudies in different cell types. A few short-term and no chronicin vivostudiesare available.Results of availablein vitrostudies indicate that the particle surface area mayplay a crucial role in the toxicity of silica. The cytotoxic activity of silica parti-cles can be related to their surface interfacing with the biological milieu ratherthan to particle size or shape (Napierskaet al.,2010). The effect related toother physico-chemical properties of SNPs are less well studied and no defi-nite conclusions can be made.Results fromin vivostudies indicate that the toxicity of SNPs can depend onnot only the material itself but also the administration route, as was shown byHudsonet al.(2008). In this study subcutaneous injection presented goodbiocompatibility, whereas intraperitoneal and intravenous injection led to fataloutcomes.Currently available data do not clarify whether amorphous SNPs - showingaugmented cytotoxicity and presumably processing oxidative DNA damagingpotential – are less or more harmful as compared with micron-sized silica(Napierskaet al.,2010).In mostin vivostudies on acute or sub-acute health effects of SNPs the ani-mals have been exposed via inhalation or intratracheal instillation. Other ex-posure routes should therefore also be checked (e.g. blood, skin, gastrointesti-nal tract). In addition chronic studies are needed to supplement and verify theexisting data.Determining the association of results fromin vitroandin vivotoxicity as-sessments is difficult; however, as concluded by Napierska et al (2010) thecommon feature seems to be cytotoxicity and inflammatory response afterexposure to SNPs.Information is so far not sufficient to clearly identify and characterize thehealth hazards of SNPs related to the different physic-chemical characteris-tics.9.6Exposure scenarios
Apart from exposure through food containing silicium dioxide, consumers'direct exposure to nano-scale SiO2is expected to be through the use of tooth-paste and cosmetic products. Oral and dermal contact are therefore the mostlikely routes of exposure unless the cosmetic products are applied as sprays.Dermal exposure to paints and inhalation of dust from grinding painted sur-faces are other potential exposures to NSPs.Furthermore it must be expected that the nanoscale fraction of microsilica inconcrete (up to 6 % w/w) will give rise to both exposure of humans by inhala-
104
tion and of the environment. However, this is not a new situation, as mi-crosilica is a naturally occurring substance, which has been used in concretesince the 1980'es.The widespread uses of SiO2in consumer products like sunscreens, surfacetreatment and paints allow for emissions of SiO2nanoparticles to the envi-ronment during and after application e.g. via wastewater or leaching ofnanoparticles from treated surfaces.9.79.7.1Risk profileEnvironment
In short-term ecotoxicity tests, silica nanoparticles has been found to exhibitthe following effects in aquatic organisms: in zebrafish there was an incidenceof jaw malformations and embryo deformities; in different algal species, cellcoagulation, membrane damage, arrested growth and decrease in chlorophyllwas found; an increased mortality was observed for Daphnia magna. Fordaphnids, SiO2concentrations of 10 mg/L caused 70% mortality (Adamset al.2006) and for algae found EC10, 72h values in the range of 11-15 mg/L werefound for two different sizes of SiO2(van Hoeckeet al.,2008). Not manystudies have analyzed dose-response relationships, thus making it difficult toperform an effects assessment following the guidelines of ECHA (2008).9.7.2Human health
Acute toxicity from oral exposure is low. There is little information is availablewith regard to toxicity from other exposure routes.In vitrostudies give someindication of a genotoxic potential although the results from different studieswere not consistent and therefore more standardized tets are required to pro-vide firm conclusions.With regard to the human health risk there is little in-formation available with regard to the specific amounts of nano SiO2used indifferent product types and thereby the extent of the exposure.Silica used in cosmetics and personal care products is in the amorphous form.In cosmetics for skin use, the silica is expected to present little, if any risk topeople. For products that might be inhaled (such as a facial powder), silicaparticles are finely ground down and may be inhaled as such. However, as-suming that no crystalline silica is present, the exposure to silica and the re-lated risk is considered low even though the inhaled particles are associatedwith respiratory toxicity.For products where silica is integrated in a matrix as in the case with sportinggoods like tennis rackets and fishing rods, no risk is identified.Conclusions from recent research into the "nanosilica hazard" states that untilnow, the health effects of SNPs have mainly been studied in terms of expo-sure via the respiratory tract, after acute or sub-acute exposure. Thereforeother exposure routes should be checked (e.g. blood, skin, gastrointestinaltract) in order to clarify if the SNP's have specific properties compared totheir bulk counterparts. It is also stated that chronic studies are needed tosupplement and verify the existing data. (Napierska et al, 2010)
105
9.8
Summary sheet for SiO2
Nanomaterial characteristicsNameSilicium dioxide, silicondioxide, silica7631-86-9SiO2White powderSource:alibaba.com
CAS numberChemical compositionAppearanceManufacturing processes
NanoSilica can be produced via for instance the sol-gel methodor high-temperature hydrolysis in a hydrogen oxygen flame. Thevapor-liquid-solid method can be used to produced silicananospring (Lidonget al.2006). Several methods can be usedproduce nanoparticles . Silica nanoparticles are often used informulation or as components of other nanoparticles. as e.g. asurface silanization may result in less cytotoxic nanoparticlessuited for bio-applications.
Nanomaterial descriptionSilica or silicon dioxide (SiO2) is an abundant mineral commonly found in nature in the form ofsand or quartz as well as in cell walls of diatoms. SiO2has a number of distinct crystalline forms(rhombohedral, hexagonal, orthorhombic) in addition to amorphous forms e.g.α-quartz, β-quartz.Amorphous nanoSilica particles can be produced in all sizes e.g. one manufacturer has nanosilicaparticles available in the size of 9, 15, 30 and 55 nm. The shape of nanosiliica is normally spherical,but other forms exsits as well such as for instance springs and nanosilica can also vary in regard totheir porousity. Nanosilica can furthermore be functionalized with instance organogroups as wellas other metals.ApplicationsSilica nanoparticles have numerous applications such as ingredients in cement, paints, solid lubri-cants, shampoos, cosmetics, face creams and food (spices). Bioelectrocemistry, curing, oil recov-ering applications and in formulation of other particles are furthermore potential application. Or-gano-silica has also been reported to be used as scratch resistant coatings. Very little informationis available about the SiO2content in the various products. Concentrations of 0.1% and 15% asbeen reported for paints, whereas organo-silica is used in concentrations of 10% in scratch resis-tant coatings Very little is known about the scale of use of nanosilica.Human health risk profileOnly fewin vivostudies are identified for silica nanoparticles. Acute toxicity from oral exposure islow. There is little information is available with regard to toxicity from other exposure routes andno dose-response relationships are established.In vitrostudies give some indication of agenotoxic potential although the results from different studies were not consistent and thereforemore standardized tets are required to provide firm conclusions. With regard to the human healthrisk there is little information available with regard to the amounts of nano SiO2 used in differentproduct types and thereby the extent of the exposure.
106
Environmental risk profileIn short-term ecotoxicity tests, silica nanoparticles has been found to exhibit the following effectsin aquatic organisms: in zebrafish there was an incidence of jaw malformations and embryo de-formities; in different algal species, cell coagulation, membrane damage, arrested growth and de-crease in chlorophyll was found; an increased mortality was observed for Daphnia magna. Fordaphnids, SiO2concentrations of 10 mg/L caused 70% mortality and for algae found EC10, 72hvalues in the range of 11-15 mg/L were found for two different sizes of SiO2. These values indicatethat nanosilica has low toxicity towards aquatic organisms. Only very few studies have analyzeddose-response relationships and estimated environmental concentrations are lacking. Therefore,no quantitative risk characterization can be made at this point in time.
107
108
10 Exposure and risk potentialBulk versus nano?A key question in relation to risk and safety assessment of nanomaterials, is towhich extent the existing knowledge base about toxicity and risk related to thebulk counterparts can be used in the evaluation of the nanomaterials and sim-ply be scaled based on the size and knowledge about possible nano-specificbehaviour and effects.As discussed in the ENHRES study (Stoneet al.,2010), and under the as-sumption that 'chemistry' is a key driver for toxicity, this would at least requirethat the chemistry should be similar for the bulk form and the nano-form ofthe material. This is however not always the case, e.g. for carbonbased nano-materials like fullerenes, where specific surface modifications are introducedin order to obtain the required properties. In addition the impact of agglom-eration and aggregation needs to be considered. For the more chemically inertparticles like TiO2and SiO2, the possibility of scaling toxicity and risk infor-mation from bulk to nano, seems to be more of an option, although it will re-quire further investigations before firm conclusions can be made. However,for materials like TiO2, SiO2and to some extent nanoclay, where naturally oc-curring, unmodified nanoscale particles have been part of some of the 'con-ventional' materials, the existing knowledge base is likely to cover some of thenanospecific properties and effects for these materials.In relation to the present survey, it is important to note that all the reviewedstudies are dealing with the core-particles, whereas most real-world uses ofengineered nanoparticles require a surface functionalisation. How these func-tionalisations/coatings affect the bioavailability, uptake / distribution / metabo-lism / excretion, and toxicity nanoparticles is at present unknown.Ecotoxicity and toxicity of nanomaterials?The bulk counterparts of the seleted nanomaterials are generally not perceivedas very hazardous chemicals, although some of them do have hazardous prop-erties that may constitute a risk in relation to certain exposure situations.The overall picture in relation to the reviewed nanomaterials is that the rangeof toxicological and ecotoxicological studies available is not sufficient to allowfirm conclusions with regard to the toxicity of the nanoparticles compared totheit bulk counterparts. This is also due to the fact that principles for physico-chemical characterization, which is a critical factor for any toxicity evaluation,still need to be further developed.The issue of characterization in the media where the nanomaterials are tested,is a frequently discussed topic in the nano(eco)toxicological literature. In thenewest studies, characterisation of the nanoparticles that are tested is mostoften carried out in terms of chemical composition, sizes, and surface area.However, it still remains a scientific challenge to characterize the test materialsin the actual test media, and hence to control the exposure. Therefore moreresearch is needed before a link between specific properties, or combinationsof properties, and observed effects can be made.
109
While reviewing the literature on environmental effects of the selected nano-materials, a lack of studies addressing degradability and accumulation becameevident – as also described by Stone et al. (2010). These two properties are asimportant as toxicity for determining the hazard profile of a material, and it istherefore recommended that more research efforts are directed towards bioac-cumulation and degradability of engineered nanomaterials.In addition, the concentrations that are used in the ecotoxicological studies aremostly much higher than the concentrations expected to be environmentallyrealistic.Another issue is that the majority of the reviewed studies have focussed onshort-term effects of the nanomaterials whereas little information is availablewith regard to long-term exposures to lower concentrations aimed at chronicendpoints.Furthermore only part of the tests performed for environmental toxicity as-sessment are aimed at the base-set organisms and the endpoints required fordoing effects assessment according to REACH as mentioned in the case offullerenes.For those substances that have been registered under REACH in 2010 (e.g.C60, Ag and TiO2) environmental data are now available for the test species inthe base set for risk assessment in REACH (i.e., fish, crustacean, algae), al-though some of these data still need replication to improve quantification ofexposure.With regard to the evaluation of toxicological effects of the nanomaterials, anissue is that most studies arein vitrostudies and only few studies are availablein vivo.However, based on available studies there are indications that a com-mon mechanism of toxicity is that exposure to nanoparticles can generate anoxidative stress response and lead to pulmonary and cardiovascular inflamma-tion. Whether other mechanisms of toxicity are of importance needs to be de-termined. However, as not all nanoparticles induce toxicity, the mechanismbehind the differences also needs clarification.Main applications and exposure?Although it may seem straight forward to determine the number of productscontaining nanomaterials, this is far from the case. Available databases con-taining information about nanomaterials are typically biased towards what issold on the internet and not necessarily what is available on the market as awhole. Nanomaterials are used in a range of products and articles not definedas “nano-based” since the nanomaterials may only constitute part of the func-tionality of the product or article e.g. suntan lotions, car catalysts or laptopcomputers.The results of the present survey indicate that titaniumdioxide, nanoclay andsiliciumdioxide are the nanomaterials used in highest volumes in products thatare also used by consumers, whereas use of cerium oxide and zero valent ironin product for consumers in Denmark has not been confirmed.Nanosilver is likely to be found in many consumer products on the Danishmarket in addition to those that are commercially available on the internet andlisted in the Woodrow Wilson database. It was not possible to quantify the
110
possible use in textiles in Denmark as part of the small industry survey, due tolack of response.In Table 4 the main consumer applications mentioned in the reviewed litera-ture for the selected nanomaterials are shown together with an indication ofthe level of environmental and consumer exposure related to their applica-tions. Applications in food and medicine are not included. The table showsthe areas of application which are considered relevant for consumers in Den-mark as well as the results from the small industry survey. It should bestressed that since this survey did not cover all potential areas of application itdoes not provide a complete picture of the current products on the marketcontaining the selected nanomaterials. Based on a primarily qualitative estima-tion, the levels of potential environmental and consumer exposure are indi-cated as:Low:Medium:High:Fixed particles in a matrix or low concentration of nanoparti-cles in the productsFree particles or direct contact/release in low or moderate con-centrationsFree particles, liquid or direct contact/release in high or mod-erate concentrations
NanomaterialFullerenes - C60
Table 4 Main applications and exposure of the selected nanomaterialsPotential environ-Potential con-Main applications (consumer)Industry survey1)mental exposuresumer exposureSportsgearCosmetics and personal careLubricants (motor oil) (~0.01 %)Cosmetics and personal care(including sun screens)PaintsPigment in mortar and cementTherapeuticSelf-cleaning ovensDiesel fuel additivePersonal careTextilesSportsgearHome and gardenFood packagingElectronics (wires and cables)CementCosmetics and personal care(including sun screens)PaintsLubricants1)
No information
Low (disposal)HighHighHighMediumMedium
LowHighHighHighMediumMedium
Titanium diox-ide - TiO2
Pigment
Zero valent iron- nZVICerium dioxide- CeO2Silver - Ag
No informationNot used as fueladditive in DKNo confirmed use
No informationNo informationHighMediumLow (disposal)MediumLow (disposal)LowMediumHighMediumMedium
No informationNo informationHighMediumLowLowLowLowMediumHighMediumMedium
Nanoclay
Leca
Silicium dioxide- SiO2
Concrete
Based on the limited industry survey carried out under the present project
The table show the estimated exposure but does not consider the actual ab-sorption by consumers through dermal exposure.
111
Risk?The question regarding the possibility of scaling of the risk from the bulk sub-stances to the nanoform, is as mentioned not yet clarified for the many differ-ent nanomaterials and will require further research. The question is furthercomplicated by the fact than many nanomaterials have surface modificationsdeliberately added to give them specific properties.For the more chemically 'inert' particles, it is more likely that behavior and ef-fects can be scaled based on knowledge about the larger particles to the nano-scale materials.With regard to human health, there are indications that some of the toxic ef-fects of nanosized TiO2can be scaled based on surface area considerationsfrom the toxicity of the micro-sized TiO2(Stoneet al.,2010). This may alsobe the case for SiO2and to some extent nanoclay. For silver, more research isrequired, although products containing nanosilver particles are likely to havebeen commercially available for more than 100 years and are thereby includedin some of the already existing toxicity test results. In addition there are someindications that the toxicity of silver is related the Ag+ ions but overall it it tooearly for firm scientific conclusions.Fullerenes in contrast are more likely to be associated with specific nano-toxicological properties, because of the very different surface characteristicscompared to the bulk material.In order to determine whether the nanomaterials give rise to new risks com-pared to the bulk materials, it is necessary to have adequate risk assessmentmethodologies that are agreed and that information is available to allow com-parison of the results from different studies across different physico-chemicalcharacteristics.The future?In order to answer the many questions regarding nanomaterials and risk moreinformation and research is required in the future. Some of the gaps can besummarized as follows:Characteristics sufficient for toxicity testingFate, behavior and kinetics of different nanoparticlesAgreement regarding risk assessment methodologies to comply withregulatory regimesMore information on chronic effects of nanomaterialsEffect of surface functionalisation on toxicity of the nanomaterials
112
Bayercoatings (2010): Dispercoll� S – Nano-silica dispersions for good initialwet strength and temperature resistance http://www.bayercoatings.de/bms/db-rsc/bms_rsc_cas.nsf/id/ADEN_Dispercoll_S(Accessed 28-11-2010)Benn, T. M.; Westerhoff, P. (2008): Nanoparticle silver released into waterfrom commercially available sock fabrics. Environ. Sci. Technol. 2008, 42(11), 4133–4139.Benn, T., Cavanagh, B., Hristovski, K., Posner, J.D., Westerhoff, P. (2010):The Release of Nanosilver from Consumer Products Used in the Home. J.Environ. Qual. 39:1875–1882.Bermudez, E., Mangum, J. B., Wong, B. A., Asgharian, B., Hext, P. M.,Warheit, D. B. and Everitt, J. I. (2004): "Pulmonary responses of mice, rats,and hamsters to subchronic inhalation of ultrafine titanium dioxide particles",Toxicol Sci, vol. 77, no. 2, pp. 347-357.Blaser, S.A.,Scheringer, M.,MacLeod, M.,Hungerbühler, K. (2008): Estima-tion of cumulative aquatic exposure and risk due to silver: Contribution ofnano-functionalized plastics and textiles. Science of The Total Environment.390, 396-409Boxall, A.B. A., Tiede, K., Chaudhry, Q. (2007): Engineered nanomaterialsin soils and water: how do they behave and could they pose a risk to humanhealth? Nanomedicine (Lond). 2007 Dec;2(6):919-27Boxall, A.B. A., Chaudhry, Q., Sinclair, C., Jones, A., Aitken, R., Jefferson,B., Watts, C. 2008. Current And Future Predicted Environmental ExposureTo Engineered Nanoparticles. York: Central Science Laboratory.Braydich-Stolle, L., Hussain, S., Schlager, J.J. and Hofmann, M.C. (2005):"Invitrocytotoxicity of nanoparticles in mammalian germline stem cells",Toxicological Sciences,vol. 88, no.2, pp. 412–419Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ,Hussain SM, Hofmann MC. (2010): Silver nanoparticles disrupt GDNF/Fynkinase signaling in spermatogonial stem cells. Toxicol Sci. 2010Aug;116(2):577-89.Briel, B. (2010): Nanoclays – Counting on Consistencyhttp://www.rockwoodadditives.com/nanoclay/pdfs/consistency.pdfBullard-Dillard, R., Creek, K.E., Scrivens, W.A. and Tour, J.M. (1996): "Tis-sue sites of uptake of 14C labelled C60", Bioorganic Chemistry, vol. 24, no. 4,pp. 376-385.Cha, K., Hong, H. W., Choi, Y. G., Lee, M. J., Park, J. H., Chae, H. K., Ryu,G., and Myung, H. (2008): "Comparison of acute responses of mice livers toshort-term exposure to nano-sized or micro-sized silver particles", Biotech-nol.Lett., vol. 30, no. 11, pp. 1893-1899.Chang, M., Kang, H. (2009): Remediation of pyrene-contaminated soil bysynthesized nanoscale zero-valent iron particles. J. Environ. Sci. Health 44,576–582Charvin, P. Abanades, P., Bechea E., Lemont, F. and Flamant, G. (2009):Hydrogen production from mixed cerium oxides via three-step water-splitting
114
cycles. Solid State Ionics, Volume 180, Issues 14-16, 25 June 2009, Pages1003-1010Chen, B.X., Wilson, S.R., Das, M., Coughlin, D.J. and Erlanger, B.F.(1998a): " Antigenicity of fullerenes: antibodies specific for fullerenes andtheir characteristics ", PNAS USA, vol. 95, no.18, pp.10809-10813.Chen, H.H., Yu, C., Ueng, T.H., Chen, S., Chen, B.J., Huang, K.J. andChiang, L.Y. 1998b, "Acute and subacute toxicity study of water-solublepolyalkylsulfonated C60 in rats", Toxicol Pathol, vol. 26, no. 1, pp.143-151.Chen, H. W., Su, S. F., Chien, C. T., Lin, W. H., Yu, S. L., Chou, C. C.,Chen, J. J. and Yang, P. C. (2006): "Titanium dioxide nanoparticles induceemphysema-like lung injury in mice", FASEB Journal, vol. 20, no. 13, pp.2393-2395.Chen, X., Mao, S.S. (2007): Titanium Dioxide Nanomaterials: Synthesis,Properties, Modifications, and Applications Chem. Rev. 2007, 107, 2891-2959Chen, C., Xing, G., Wang, J., Zhao, Y., Li, B., Tang, J., Jia, G., Wang, T.,Sun, J., Xing, L., Yuan, H., Gao, Y., Meng, H., Chen, Z., Zhao. F, Chai, Z.and Fang, X. (2005): "Multihydroxylated [Gd@C82(OH)22]n nanoparticles:antineoplastic activity of high efficiency and low toxicity", Nano Letters, vol.5, no.10, pp. 2050-2057.Chen Z, Meng H, Xing G, Yuan H, Zhao F, Liu R, Chang X, Gao X, WangT, Jia G, Ye C, Chai Z, Zhao Y. (2008): Age-related differences in pulmo-nary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxic-ity has susceptible population. Environ Sci Technol. 2008 Dec1;42(23):8985-92.Cho WS, Choi M, Han BS, Cho M, Oh J, Park K, Kim SJ, Kim SH, Jeong J.(2007): Inflammatory mediators induced by intratracheal instillation of ul-trafine amorphous silica particles. Toxicol Lett. 2007 Dec 10;175(1-3):24-33.Epub 2007 Sep 29.Cho, M., Cho, W.S., Choi, M., Kim, S.J., Han, B.S., Kim, S.., Kim, H.O.,Sheen, Y.Y., and Jeong, J. (2009): The impact of size on tissue distributionand elimination by single intravenous injection of silica nanoparticles. ToxicolLett, 189(3):177-183. PubMed abstract Internet address:http://www.ncbi.nlm.nih.gov/pubmed/19397964. Last accessed on December20, 2010.Choi, O. and Hu, 7. (2008): “Impact of Silver Nano-particles on WastewaterTreatment”, ACWA Annual Conference Presentation, Department of Civiland Environmental Engineering, University of Missouri, Columbia, USA.Christensen FM, Johnston HJ, Stone V, Aitken RJ, Hankin S, Peters S,Aschberger K. (2010): Nano-silver - feasibility and challenges for humanhealth risk assessment based on open literature. Nanotoxicology. 2010Sep;4(3):284-95.Chung, T.H., Wu, S.H., Yao, M., Lu, C.W., Lin, Y.S., Hung, Y., Mou,C.Y., Chen, Y.C., and Huang, D.M. (2007): The effect of surface charge onthe uptake and biological function of mesoporous silica nanoparticles in 3t3-l1cells and human mesenchymal stem cells. Biomaterials, 28(19):2959-2966.
115
Abstract from PubMed 17397919. PubMed abstract Internet address:http://www.ncbi.nlm.nih.gov/sites/entrez?orig_db=PubMed&db=pubmed&cmd=Search&TransSchema=title&term=17397919. Last accessed on Decem-ber 20, 2010.Consumer Project No. 81 (2007): Stuer-Lauridsen, F., Kamper, A., BorlingP., Petersen, G.I., Hansen, S.F., Baun, A. Mapping of products containingnanoparticles or based on nanotechnology, Danish EPA. Available:http://www2.mst.dk/common/Udgivramme/Frame.asp?pg=http://www2.mst.dk/Udgiv/publications/2007/978-87-7052-536-7/html/default_eng.htmCupi, D. (2010): Review on the Environmental Hazards and Human HealthEffects of Silica Nanoparticles. Individual project, MSc EnvironmentalChemistry and Health, DTU.Damtoft, J.S. (2010): Personal communication with Jesper Sand Damtoft,Ålborg Portland, Nov. 2010.Danmarks Statistik (2010):http://www.statistikbanken.dk/statbank5a/default.asp?w=1280, , Nov. 2010.Dantonit (2010): http://www.dantonit.dk/Default.aspx?ID=24Das, M., Patil, S., Bhargava, N., Kang, J. F., Riedel, L. M., Seal, S. andHickman, J. J. (2007): "Auto-catalytic ceria nanoparticles offerneuroprotection to adult rat spinal cord neurons", Biomaterials, vol. 28, no.10, pp. 1918-1925.Deshpande S., Patil S., Kuchibhatla S.V.N.T. and Seal S., 2005: Sizedependency variation in lattice pa-rameter and valency states innanocrystalline cerium oxide, Applied Physics Letters, 87, 133113, AmericanInstitute of PhysicsDriscoll, K. E., Deyo, L. C., Carter, J. M., Howard, B. W., Hassenbein, D. G.and Bertram, T. A. (1997): "Effects of particle exposure and particle-elicitedinflammatory cells on mutation in rat alveolar epithelial cells", Carcinogenesis,vol. 18, no.2, pp. 423-430.ECHA (2008): Guidance on information requirements and chemical safetyassessment Chapter R.7b: Endpoint specific guidance May 2008 (version1.1), European Chemicals Agency.Environmental Defense (ED) and Dupont (2007): Nanomaterial risk assess-ment worksheet: DuPont™ light stabilizer for use as a polymer additive.Washington DC: Environmental Defense - Dupont Nano Partnership.http://nanoriskframework.org/page.cfm?tagID=1326.Environmental Project No. 1206 (2007): Tønning, K., Poulsen, M.Nanotechnology in the Danish industry - survey on production and applica-tion. Danish Ministry of the Environment Danish Environmental ProtectionAgency, 2007.EOF (2010): Personal communication with Michael Mücke Jensen, Energi-og Olieforum, Danmark, Dec. 2010.EPA (2007): Nanotechnology White Pater. Science Policy Council. UnitedStates Environmental Protection Agency, EPA 100/B-07/001 February 2007
116
Eriksen (2010): Personal communication with Kirsten Eriksen, COWI A/S,Dec. 2010.Erlanger, B.F., Chen, B.X., Zhu, M. and Brus, L. (2001): "Binding of an anti-fullerene IgG monoclonal antibody to single wall carbon nanotubes", NanoLetters, vol. 1, no.9, pp. 465-467.Espindola-Gonzalez A., Martinez-Hernandez A. L., Angeles-Chavez C.,Castano V. M., Velasco-Santos C., (2010): Novel Crystalline SiO2Nanoparticles via Annelids Bioprocessing of Agro-Industrial Wastes.Nanoscale Res Lett 5:1408–1417.Everest (2010):http://www.everest.com.tw/_english/11_star_product/01_edit.aspx?ID=8],Dec. 2010Farré, M., Péreza, S., Gajda-Schrantz, K., Osorio, V., Kantiani, L.,Ginebreda A., Barceló, D. (2010): First determination of C-60 andC(70)fullerenes and N-methylfulleropyrrolidine C-60 on the suspended material ofwastewater effluents by liquid chromatography hybrid quadrupole linear iontrap tandem mass spectrometry. Journal of Hydrology, 383 (1-2), 44 -51.Federici, G., Shaw, B.J. and Handy, R.D. (2007): "Toxicity of titaniumdioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury,oxidative stress, and other physiological effects", Aquatic Toxicology, vol. 84,pp. 415-430.Fent K., Weisbrod C.J., Wirth-Heller A., Pieles U., (2010): Assessment ofuptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio re-rio) early life stages. Aquat Toxicol. 100(2):218-28.Ferin, J., Oberdorster, G. and Penney, D. P. (1992): "Pulmonary retention ofultrafine and fine particles in rats", Am J Respir Cell Mol Biol, vol. 6, no.5,pp. 535-542.Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Møller P. (2009):Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenesand single-walled carbon nanotubes. Environ Health Perspect. 2009May;117(5):703-8. Epub 2008 Nov 12.Franco, A., Hansen, S.F., Olsen, S.I., Butti, L. (2007): Limits and Prospectsof the “Incremental Approach” and the European Legislation on the Man-agement of Risks related to Nanomaterials. Regulatory Toxicology and Phar-macology 48: 171-183.Fujita, K., Morimoto, Y., Ogami, A., Myojyo, T., Tanaka, I., Shimada, M.,Wang, W.N., Endoh, S., Uchida, K., Nakazato, T., Yamamoto, K., Fukui,H., Horie, M., Yoshida, Y., Iwahashi, H. and Nakanishi, J. (2009): "Gene ex-pression profiles in rat lung after inhalation exposure to C(60) fullerene parti-cles" Toxicology, vol. 258, no. 1, pp. 47-55.Geranio, L., Heuberger, M., Nowack, B. (2010): The behaviour of silvernanotextiles during washing. Environ Sci Technol, 43, 8116-8118.Gharbi, N., Pressac, M., Hadchouel, M., Szwarc, H., Wilson, S.R. andMoussa, F. (2005): "[60]Fullerene is a powerful antioxidant in vivo with noacute or subacute toxicity" Nano Letters, vol. 5, no. 12, pp. 2578-2585.
117
Gonzalez, L., Sanderson, B.J.S., Kirsch-Volders, (2011): Adaptations of thein vitro MN assay for the genotoxicity assessment of nanomaterials.Mutagenesis vol. 26 no. 1 pp. 185–191, 2011Gottschalk, F.,Scholz, R.W.,Nowack, B. (2010a): Probabilistic material flowmodeling for assessing the environmental exposure to compounds: Methodol-ogy and an application to engineered nano-tio2 particles. EnvironmentalModelling & Software. 25, 320-332.Gottschalk, F.,Sonderer, T.,Scholz, R.W.,Nowack, B. (2010b): Possibilitiesand limitations of modeling environmental exposure to engineered nanomate-rials by probabilistic material flow analysis. Environmental Toxicology andChemistry. 29, 1036-1048.Grassian, V. H., Adamcakova-Dodd, A., Pettibone, J. M., O'shaughnessy, P.T. and Thorne, P. S. (2007): "Inflammatory response of mice to manufac-tured titanium dioxide nanoparticles: comparison of size effects through dif-ferent exposure routes", Nanotoxicol, vol. 1, no. 3, pp. 211-226Grieger, K., Fjordbøge, A., Hartmann, N.B., Eriksson, E., Bjerg, P.L., Baun,A. (2010): Environmental benefits and risks of zero-valent iron nanoparticles(nZVI) for in situ remediation: risk mitigation or trade-off? Journal of Con-taminant Hydrology, 118: 165-183.Griffitt, R.J., Luo, J., Gao, J., Bonzongo, J.C. and Barber, D.S. (2008): "Ef-fects of particle composition and species on toxicity of metallic nanomaterialsin aquatic organisms", Environmental Toxicology and Chemistry, vol. 27, no.9, pp. 1972-1978.Gulbranson SH, Hud JA, Hansen RC. (2000): Argyria following the use ofdietary supplements containing colloidal silver protein. Cutis 66:373_376.Ha SW., Camalier C.E., Beck G.R. Jr., Lee JK., (2009): New method to pre-pare very stable and biocompatible fluorescent silica nanoparticles. ChemCommun (Camb). 28(20):2881–2883. doi:10.1039/b902195g.Hansen, S.F., Michelson, E., Kamper, A., Borling, P., Stuer-Lauridsen, F. &Baun, A. (2008): Categorization framework to aid exposure assessment ofnanomaterials in consumer products. Ecotoxicology 17 (5): 438-447.Harper, S., Usenko, C., Hutchinson, J.E., Maddux, B.I.S., Tanguay, R.L.(2008):In vivobiodistribution and toxicity depends on nanomaterial compo-sition, size, surface functionalisation and routses of exposure. J. ExperimentalNanoscience, vol. 3, no. 3, 195-206.Velzeboer, I., Hendriks, A.J., Ragas,A.M.J. and Van de Meent, D. 2008, "Aquatic ecotoxicity tests of some nano-materials", Environmental Toxicology and Chemistry, vol. 27, no. 9, pp.1942-1947.Hartmann, N.B., Von der Kammer, F., Hofmann, T., Baalousha, M., Ot-tofuelling, S., Baun, A. (2010): Algal testing of titanium dioxide nanoparti-cles—Testing considerations, inhibitory effects and modification of cadmiumbioavailability. Toxicology 269 (2010) 190–197Health and Safety Executive (2004): Health effects of particles produced fornanotechnologies HSE Hazard assessment document EH75/6 December.London: HSE.
118
Heinlaan, M., Ivask, A., Blinova, I., Dubourguier, H.C. and Kahru, A.(2008): "Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vi-brio fischeri and crustaceans Daphnia magna and Thamnocephalus platyu-rus", Chemosphere, vol. 71, no. 7, pp. 1308-1316.Henry TB, Menn FM, Fleming JT, Wilgus J, Compton RN, Sayler GS.(2007): Attributing effects of aqueous C60 nano-aggregates to tetrahydrofu-ran decomposition products in larval zebrafish by assessment of gene expres-sion. Environ Health Perspect. 2007 Jul;115(7):1059-65.Hill WR, Pillsbury DM. (1939): Argyria, the pharmacology of silver. Balti-more, MD, Williams and Wilkins.Huczko, A., Lange, H. and Calko, E. (1999): "Fullerenes: experimental evi-dence for a null risk of skin irritation and allergy", Fullerene Sci Technol, vol.7, no. 5, pp. 935-939.Hudson SP, Padera RF, Langer R, Kohane DS. (2008): The biocompatibilityof mesoporous silicates. Biomaterials. 2008 Oct;29(30):4045-55.Hyun, J. S., Lee, B. S., Ryu, H. Y., Sung, J. H., Chung, K. H., and Yu, I. J.2008, "Effects of repeated silver nanoparticles exposure on the histologicalstructure and mucins of nasal respiratory mucosa in rats", Toxicol Lett, vol.182, no. 1-3, pp. 24-28.Imada, S., Yamanaka, N., Tamai, S. (2008): Water table depth affects Popu-lus alba fine root growth andwhole plant biomass. Funct. Ecol. 22, 1018–1026.Inoue, K., Takano, H., Ohnuki, M., Yanagisawa, R., Sakurai, M., Shimada,A., Mizushima, K. and Yoshikawa, T. (2008): "Size effects of nanomaterialson lung inflammation and coagulatory disturbance", Int J ImmunopatholPharmacol, vol. 21, no. 1, pp. 197-206.Intersport (2010): Personal communication with Intersport. Dec. 2010.Jensen, I. (2010): Personal communication with Ib Jensen, Unicon, Nov.2010.Ji, J.H., Jung, J.H., Kim, S.S., Yoon, J.U., Park, J.D., Choi, B.S., Chung, Y.H.,Kwon, I.H., Jeong, J., Han, B.S., Shin, J.H., Sung, J.H., Song, K.S. and Yu,I.J. 2007, "Twenty-eight-day inhalation toxicity study of silver nanoparticles insprague-dawley rats", Inhalation Toxicology, vol. 19, no. 10, pp. 857-871.Jin, C. Y., Zhu, B. S., Wang, X. F. and Lu, Q. H. (2008): "Cytotoxicity oftitanium dioxide nanoparticles in mouse fibroblast cells", Chemical Researchin Toxicology, vol. 21, no. 9, pp. 1871-1877.Johansen A, Pedersen AL, Jensen KA, Karlson U, Hansen BM, Scott-Fordsmand JJ, Winding A. (2008): Effects of C60 fullerene nanoparticles onsoil bacteria and protozoans. Environ Toxicol Chem. 2008 Sep;27(9):1895-903.Kaegi R., Ulrich A., Sinnet B., Vonbank R., Wichser A., Zuleeg S., SimmlerH., Brunner S., Vonmont H., Burkhardt M. and Boller M. (2008): SyntheticTiO2nanoparticle emission from exterior facades into the aquatic environ-ment, Environ. Pollut., 2008, 156, 233–239.
119
Kaegi, R., Sinnet, B., Zuleeg, S., Hagendorfer, H., Mueller, E., Vonbank, R.,Boller, M., Burkhardt, M. (2010):Release of silver nanoparticles from outdoorfacades. Environ Pollut, 158 (9), 2900-2905.Kaewamatawong T., Shimada A., Okajima M., Inoue H., Morita T., InoueK., Takano H., (2006): Acute and subacute pulmonary toxicity of low dose ofultrafine colloidal silica particles in mice after intratracheal instillation. ToxicolPathol (34)7:958-965.Kato S, Aoshima H, Saitoh Y, Miwa N. (2009): Biological safety of Lipo-Fullerene composed of squalane and fullerene-C60 upon mutagenesis, photo-cytotoxicity, and permeability into the human skin tissue. Basic Clin Pharma-col Toxicol. 2009 Jun;104(6):483-7.KemI (2009): The use of nanomaterials in Sweden 2008 - analysis and prog-nosis. Swedish Chemicals Agency. KemI PM 1/09.Keenan CR, Goth-Goldstein R, Lucas D, Sedlak DL. (2009): Oxidativestress induced by zero-valent iron nanoparticles and Fe(II) in human bron-chial epithelial cells. Environ Sci Technol. 2009 Jun 15;43(12):4555-60.Kim YS, Kim JS, Cho HS, Rha DS, Park JD, Choi BS, Lim R, Chang HK,Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ. (2008): Twenty-eight-day oraltoxicity, genotoxicity, and gender-related tissue distribution of silver nanopar-ticles in Sprague-Dawley rats. Inhal. Toxicol. 20: 575–583.Kiss, B., Biro, T., Czifra, G., Toth, B. I., Kertesz, Z., Szikszai, Z., Kiss, A. Z.,Juhasz, I., Zouboulis, C. C. and Hunyadi, J. (2008): "Investigation of mi-cronized titanium dioxide penetration in human skin xenografts and its effecton cellular functions of human skin-derived cells", Exp Dermatol, vol. 17, no.8, pp. 659-667.Komatsu, T., Tabata, M., Kubo-Irie, M., Shimizu, T., Suzuki, K.I., Nihei, Y.and Takeda K. (2008): "The effects of nanoparticles on mouse testis Leydigcells in vitro", Toxicol in Vitro, vol. 22, no. 8, pp. 1825–1831.Kristensen, H.V., Vinding, K.F., Grieger, K.D., Hansen., S.F. (2009):Adopting Eco-Innovation in Danish Polymer Industry Working withNanotechnology: Drivers, Barriers and Future Strategies, for the Nanotech-nology Law and Business 6(3):416-440.Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, PrasadPN. (2010):In vivobiodistribution and clearance studies using multimodalorganically modified silica nanoparticles. ACS Nano. 2010 Feb 23;4(2):699-708.Larese, F.F., D'Agostin, F., Crosera, M., Adami, G., Renzi, N., Bovenzi ,M.and Maina, G. (2009): "Human skin penetration of silver nanoparticlesthrough intact and damaged skin",Toxicol,vol. 225, no. 1-2, pp.33-37.K. P. Lee and D. P. Kelly (1992): The pulmonary response and clearance ofLudox colloidal silica after a 4-week inhalation exposure in rats. Fundamentaland Applied Toxicology, Volume 19, Issue 3, October 1992, Pages 399-410Lee, C., Kim, J.Y., Lee, W.I., Nelson, K.L., Yoon, J., Sedlak, D.L. (2008):Bactericidal effect of zero-valent iron nanoparticles on escherichia coli.Environ. Sci. Technol. 42, 4927–4933.
120
Li, X., Elliott, D.W., Zhang, W. (2006): Zero-valent iron nanoparticles forabatement of environmental pollutants: materials and engineering aspects.Crit. Rev. Solid State Mater. Sci. 31, 11–22.Li, H., Zhou, Q., Wu, Y., Fu, J., Wang, T., Jiang, G. (2009): Effects of wa-terborne nano-iron on medaka (oryzias latipes): antioxidant enzymatic activ-ity, lipid peroxidation and histopathology. Ecotoxicol. Environ. Saf. 72, 684–692.Li PR, Wei JC, Chiu YF, Su HL, Peng FC, Lin JJ. (2010): Evaluation on cy-totoxicity and genotoxicity of the exfoliated silicate nanoclay. ACS Appl Ma-ter Interfaces. 2010 Jun;2(6):1608-13.Lide D.R., ed. (2009): CRC Handbook of Chemistry and Physics, 89th Edi-tion, CRC Press/Taylor and Fran-cis, Boca Raton, FloridaLidong Wang, D Major, P Paga, D Zhang, M G Norton, D N McIlroy(2006): "High yield synthesis and lithography of silica-based nanospringmats". Nanotechnology 17: S298–S303Lin, W., Huang, Y. W., Zhou, X. D. and Ma, Y. (2006): "Toxicity of ceriumoxide nanoparticles in human lung cancer cells", Int J Toxicol, vol. 25, no.6,pp. 451-457.Lindgreen, H. (2010): Personal communication with Holger Lindgreen,Geus, Nov. 2010.Lison, D., Kirsch-Volders, M., Hoet, P.. Martens, J., Kirschhock, C. (2009):Physico-chemical determinants of toxicity : a rational approach towards safernanostructured materials - S�NANO”. Final Report Phase 1. Brussels : Bel-gian Science Policy 2009 – 18 p. (Research Programme Science for a Sus-tainable Development)Lovern SB, Klaper R. (2006): Daphnia magna mortality when exposed totitanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem.;2006 Apr;25(4):1132-7.Lovern SB, Strickler JR, Klaper R. (2007): Behavioral and physiologicalchanges in Daphnia magna when exposed to nanoparticle suspensions (tita-nium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol. 2007Jun 15;41(12):4465-70.Lowry, G. V. and Casman E. A. (2009): Nanomaterial Transport, Transfor-mation, and Fate ion the Environment: A Risk-based Perspective on ResearchNeeds. In Risk, Uncertainty and Decision Analysis for Nanomaterials: Envi-ronmental Risks and Benefits and Emerging Consumer Products. Eds. IgorLinkov and Jefferey Stevens. Springer Verlag. pp. 125-139.Luoma, S. N. (2008): Silver Nanotechnologies and the Environment OldProblems or New Challenges? PEN 15. Washington, DC: Project on Emerg-ing Nanotechnologies, Woodrow Wilson International Center for Scholars.Makimura D., Metin C., Kabashima T., Matsuoka T., Nguyen Q. P.,Miranda C.R. (2010): Combined modeling and experimental studies of hy-droxylated silica nanoparticles. J Mater Sci 45:5084–5088.
121
Mknano (2010): mkNANO.http://www.mknano.com/searchresultsproduct.asp (Accessed 28-11-2010)Mori, T., Takada, H., Ito, S., Matsubayashi, K., Miwa, N. and Sawaguchi, T.(2006): "Preclinical studies on safety of fullerene upon acute oral administra-tion and evaluation for no mutagenesis", Toxicology, vol. 225, no. 1, pp. 48-54.Morsy, M.S., Alsayed, S.H. and Aquel, M. (2010): Effect of Nano-clay onMechanical Properties and Microstructure of Ordinary Portland CementMortar. International Journal of Civil & Environmental Engineering IJCEE-IJENS Vol: 10 No:01Mueller, N.C., Nowack, B. (2008): Exposure modeling of engineerednanoparticles in the environment. Environmental Science & Technology. 42,4447-4453.Nano-C (2002): Masters of Flame: Industrial Production of Fullerenes Be-comes a Reality. Nano-C Inc., Westwood, MA.Nanocor (2010): Nanocor. http://www.nanocor.com/Nanowerk (2010): Nanomaterial Database Search.http://www.nanowerk.com/phpscripts/n_dbsearch.php (Accessed 5 Novem-ber, 2010).Naota M, Shimada A, Morita T, Inoue K, Takano H. (2009): Translocationpathway of the intratracheally instilled C60 fullerene from the lung into theblood circulation in the mouse: possible association of diffusion and caveolae-mediated pinocytosis. Toxicol Pathol. 2009;37(4):456-62. Epub 2009 Apr 3.Napierska, D., Thomassen, Leen CJ., Lison, Dominique, Martens, Johan A.,Hoet, Peter H. (2010): The nanosilica hazard: another variable entity. Particleand Fibre Toxicology 7:39http://www.particleandfibretoxicology.com/content/7/1/39Nelson S.M., MahmoudT., Beaux II M., Shapiro P., McIlroy D.N.,Stenkamp D.L., (2010): Toxic and teratogenic silica nanowires in developingvertebrate embryos. Nanomedicine 6(1): 93–102.doi:10.1016/j.nano.2009.05.003.NFA (2010): Nanoplast - Nanoteknologiske materialer og produkter i plast-industrien. Baggrund. Det Nationale Forskningscenter for Arbejdsmiljø.http://www.arbejdsmiljoforskning.dk/da/projekter/nanoteknologiske-materialer-og-produkter-i-plastindustrien---nanoplast/baggrundNike (2010): Personal communication with Ms. Verena Janowski, Nike Den-mark. Dec. 2010.Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K. (2009):Influence of 70 nm silica particles in mice with cisplatin or paraquat-inducedtoxicity. Pharmazie. 2009 Jun;64(6):395-7.Nordberg, G.F. and L. Gerhardsson (1988): Silver. In: Handbook on Toxic-ity of Inorganic Compounds, H.G. Seiler and H. Sigel, eds. Marcel Dekker,Inc., New York, pp. 619-623.
122
Norwegian Labour Inspection Authority (2010): Survey on Production , Im-port and Use of Nanotechnological Products in Norway. Final Report, July2010Nowack B., Krug H., Height M. (2011): 120 Years of Nanosilver History:Implications for Policy Makers. Environ Sci Technol. 2011 Mar 2. [Epubahead of print] NTP, 2009. Chemical information Review Document for Sil-ica flour. Micronised-quartz.Supporting Nomination for ToxicologicalEvaluation by the national Toxicology Program.Oberdörster, E., Larkin, P., Rogers, J. (2006): Rapid environmental impactscreening for engineered nanomaterials: a case study using microarray tech-nology. Project on Emerging Nanotechnologies, Woodrow Wilson Interna-tional Center for Scholars, June 2006, Washington D.C.Park B, Martin P, Harris C, Guest R, Whittingham A, Jenkinson P, HandleyJ. (2007): Initial in vitro screening approach to investigate the potential healthand environmental hazards of Enviroxtrade mark - a nanoparticulate ceriumoxide diesel fuel additive. Part Fibre Toxicol. 2007 Dec 5;4:12.Park, Barry, Donaldson, Kenneth, Duffin, Rodger, Tran, Lang, Kelly, Frank,Mudway, Ian, Morin, Jean-Paul, Guest, Robert, Jenkinson, Peter, Samaras,Zissis, Giannouli, Myrsini, Kouridis, Haris and Martin, Patricia (2008): Haz-ard and Risk Assessment of a Nanoparticulate Cerium Oxide-Based DieselFuel Additive—A Case. Study. Inhalation Toxicology, 20:6, 547 — 566Park, E. J., Choi, J., Park, Y. K., and Park, K. (2008a): "Oxidative stress in-duced by cerium oxide nanoparticles in cultured BEAS-2B cells", Toxicol,vol. 245, no. 1-2, pp. 90-100.Park EJ, Park K., (2009): Oxidative stress and pro-inflammatory responsesinduced by silica nanoparticles in vivo and in vitro. Toxicology Letters184(1):18-25.Park YH, Kim JN, Jeong SH, Choi JE, Lee SH, Choi BH, Lee JP, Sohn KH,Park KL, Kim MK, Son SW. (2010): Assessment of dermal toxicity ofnanosilica using cultured keratinocytes, a human skin equivalent model and anin vivo model. Toxicology. 2010 Jan 12;267(1-3):178-81. Epub 2009 Oct 20.Patil S, Sandberg A, Heckert E, Self W, Seal S. (2007): Protein adsorptionand cellular uptake of cerium oxide nanoparticles as a function of zeta poten-tial. Biomaterials. 2007 Nov;28(31):4600-7. Epub 2007 Aug 1.PEN (2011): The Project on Emerging Nanotechnologies. Consumer Prod-uct Inventory. Woodrow Wilson International Center for Scholars. AccessedJanuary 2011: http://www.nanotechproject.org/inventories/consumer/Phenrat T, Long TC, Lowry GV, Veronesi B. (2009): Partial oxidation ("ag-ing") and surface modification decrease the toxicity of nanosized zerovalentiron. Environ Sci Technol. 2009 Jan 1;43(1):195-200.Pirngadi, P., Richter, S. (2005): New Developments in the Application ofNanoclays for HFFR Cable Compounds. Proceedings of the 54thIWCS/Focus Conference, 2005.http://ecadigitallibrary.com/pdf/IWCS05/IWCS0582_lke.pdf
123
Powell JJ, Faria N, Thomas-McKay E, Pele LC. (2010): Origin and fate ofdietary nanoparticles and microparticles in the gastrointestinal tract. J Auto-immun. 2010 May;34(3):J226-33. Epub 2010 Jan 21.Pronk, M. E. J.; Wijnhoven, S. W. P.; Bleeker, E. A. J.; Heugens, E. H. W.;Peijnenburg, W. J. G. M.; Luttik, R.; Hakkert, B. C. (2009): Nanomaterialsunder REACH: Nanosilver as a case study. National Institute for PublicHealth and the Environment, Bilthoven, the NetherlandsProspect (2010): Global Nanomaterials Safety. Toxicological Review of NanoCesium Oxide. Ecotoxicology Test Protocols for Representative Nanomateri-als in Support of the OECD Sponsorship Programme. 1 July 2010Ramsden, C.S., Smith, T.J., Shaw, B.J., Handy, R.D. (2009): Dietary expo-sure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchusmykiss): no effect on growth, but subtle biochemical disturbances in the brain.Ecotoxicology 18:939–951.Ratte, H. T. (1999): Bioaccumulation and toxicity of silver compounds: Areview. Environmental Toxicology and Chemistry, 18: 89–108.Regulation (EC) No 1272/2008 of the European Parliament and of theCouncil of 16 December 2008 on classification, labelling and packaging ofsubstances and mixtures, amending and repealing Directives 67/548/EEC and1999/45/EC, and amending Regulation (EC) No 1907/2006.Regulation (EC) No 1907/2006 of the European Parliament and of theCouncil of 18 December 2006 concerning the Registration, Evaluation, Au-thorisation and Restriction of Chemicals (REACH), establishing a EuropeanChemicals Agency, amending Directive 1999/45/EC and repealing CouncilRegulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94as well as Council Directive 76/769/EEC and Commission Directives91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.RIVM (2009): "Nanomaterials under REACH: Nanosilver as a case study",RIVM Report 601780003/2009Roursgaard, M., Poulsen, S.S., Kepley, C.L., Hammer, M., Nielsen, G.D.and Larsen, S.T. (2008): "Polyhydroxylated C60 fullerene (fullerenol) at-tenuates neutrophilic lung inflammation in mice", Bas Clin Pharmacol Toxi-col, vol. 103, no. 4, pp. 386-388.Saleh, N.B., Pfefferle, L.D. and Elimelech, M. (2008): “Aggregation kineticsof multiwalled carbon nanotubes in aquatic systems: measurements and envi-ronmental implications”, Environmental Science and Technology, vol. 42, pp.7963-9.Saleh, N., Kim, H., Phenrat, T., Lowry, G.V., Tilton, R.D., Matyjaszewski,K., Lowry, G.V., Tilton, R.D. (2008): Ionic strength and composition affectthe mobility of surface-modified Fe0 nanoparticles in water-saturated sandcolumns. Environ. Sci. Technol. 42, 3349–3355.Sayes, C.M., Fortner, J.D., Guo, W., Lyon, D.Y., Boyd, A.M., Ausman, K.,Tao, Y.J., Sitharaman, B., Wilson, L.J., Hughes, J.B., West, J.L. and Colvin,V.L. (2004): "The differential cytotoxicity of water-soluble fullerenes", NanoLetters, vol. 4, no. 10, pp. 1881-1887.
124
Sayes, C.M., Marchione, A.A., Reed, K.L. and Warhei,t D.B. (2007): "Com-parative pulmonary toxicity assessments of C60 water suspensions in rats: fewdifferences in fullerene toxicity in vivo in contrast to in vitro profiles", NanoLetters, vol. 7, no. 8, pp. 2399-2406.Sayes CM, Reed KL, Glover KP, Swain KA, Ostraat ML, Donner EM,Warheit DB. (2010): Changing the dose metric for inhalation toxicity studies:Short-term study in rats with engineered aerosolized amorphous silicananoparticles. Inhalation Toxicology, 22(4): 348-354 (March 2010).SCENIHR (2005): Opinion on the appropiateness of existing methodologiesto access the potential risks associated with engineered and adventitious prod-ucts of nanotechnologies. Brussels: European Commision, health and con-sumer protection directorate-general, Scientific Committee on Emerging andnewly Identified Health Risks (SCENIHR)Schreiner KM, Filley TR, Blanchette RA, Bowen BB, Bolskar RD, HockadayWC, Masiello CA, Raebiger JW. (2009): White-rot basidiomycete-mediateddecomposition of C60 fullerol. Environ Sci Technol. 2009 May1;43(9):3162-8.Schrick, B., Hydutsky, B.W., Blough, J.L., Mallouk, T.E. (2004): Deliveryvehicles for zerovalent metal nanoparticles in soil and groundwater. Chem.Mater. 16, 2187–2193.Scott-Fordsmand JJ, Krogh PH, Schaefer M, Johansen A. (2008): The toxic-ity testing of double-walled nanotubes-contaminated food to Eisenia venetaearthworms. Ecotoxicol Environ Saf. 2008 May 29.Scown, T.M., van Aerle, R., Johnston, B.D., Cumberland, S., Lead, J.R.,Owen, R. & Tyler, C.R. (2009): "High Doses of Intravenously AdministeredTitanium Dioxide Nanoparticles Accumulate in the Kidneys of RainbowTrout but with no Observable Impairment of Renal Function", ToxicologicalSciences, vol. 109, no. 2, pp. 372-380.Sera, N., Tokiwa, H. and Miyata, N. (1996): "Mutagenicity of the fullereneC60-generated singlet oxygen dependent formation of lipid peroxides", Car-cinogenesis, vol. 17, no. 10, pp. 2163-2169.Sharma AK, Schmidt B, Frandsen H, Jacobsen NR, Larsen EH, BinderupML. (2010): Genotoxicity of unmodified and organo-modified montmorillo-nite. Mutat Res. 2010 Jul 19;700(1-2):18-25. Epub 2010 Apr 28.Shinohara N, Matsumoto K, Endoh S, Maru J, Nakanishi (2009): J.In vitroandin vivogenotoxicity tests on fullerene C60 nanoparticles. Toxicol Lett.2009 Dec 15;191(2-3):289-96. Epub 2009 Sep 20.Schubert D, Dargusch R, Raitano J, Chan SW. (2006): Cerium and yttriumoxide nanoparticles are neuroprotective. Biochem Biophys Res Commun.2006 Mar 31;342(1):86-91. Epub 2006 Feb 3.Silver S. (2003): Bacterial silver resistance: Molecular biology and uses andmisuses of silver compounds. FEMS Microbiol Rev 27:341_353.Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG,Wright CJ, Doak SH. (2009): Nanogenotoxicology: The DNA damaging po-
125
tential of engineered nanomaterials. Biomaterials. 2009 Aug;30(23-24):3891-914. Epub 2009 May 8.Sitharaman, B., Wilson, L. J., Hughes, J. B., West, J. L., Colvin, V. L. (2004):The differential cytotoxicity of water-soluble fullerenes. Nano Lett 4(10):1881-1887.So SJ, Jang IS, Han CS. (2008): Effect of micro/nano silica particle feedingfor mice. J Nanosci Nanotechnol. 2008 Oct;8(10):5367-71.Stone, V., Hankin, S., Aitken, R., Aschberger, K., Baun, A., Christensen, F.,Fernandes, T., Hansen, S.F., Hartmann, N.B., Hutchinson, G., Johnston, H.,Micheletti, G., Peters, S., Ross, B., Sokull-Kluettgen, B., Stark, D., Tran, L.(2010): Engineered Nanoparticles: Review of Health and EnvironmentalSafety (ENRHES). Available:http://nmi.jrc.ec.europa.eu/project/ENRHES.htm (Accessed February 1,2010).Suha W.H., Suslickb K.S., Stuckya G.D., Suh Y-H., (2009): Nanotechnol-ogy, nanotoxicology, and neuroscience. Prog Neurobiol. 87(3):133–170.doi:10.1016/j.pneurobio.2008.09.009.Sun, H., Wang, L., Zhang, R., Sui, J., Xu, G. (2006): Treatment of ground-water polluted by arsenic compounds by zero valent iron. J. Hazard. Mater.129, 297–303.Sun W., Wang D., Zhai Z., Gao R., Jiao K., (2009): Direct Electrochemistryof Hemoglobin Immobilized in the Sodium Alginate and SiO2 NanoparticlesBionanocomposite Film on a Carbon Ionic Liquid Electrode. J. Iran. Chem.Soc, 6(2): 412-419Sung J.H., Ji J.H., Yoon J.U., Kim D.S., Song M.Y., Jeong J., Han B.S., HanJ.H., Chung Y.H., Kim J., Kim T.S., Chang H.K., Lee E.J., Lee J.H., Yu I.J.,(2008): Lung function changes in Sprague-Dawley rats after prolonged inha-lation exposure to silver nanoparticles.Inhal Toxicol20:567-574.Sung, J.H., Ji, J.H., Park, J.D., Yoon, J.U., Kim, D.S., Jeon, K.S., Song, M.Y.,Jeong, J., Han, B.S., Han, J.H., Chung, Y.H., Chang, H.K., Lee, J.H., Cho,M.H., Kelman, B.J. and Yu, I.J. (2009): "Subchronic inhalation toxicity ofsilver nanoparticles", Toxicol Sciences, vol. 108, no.2, 452-461.Suresh, R., Borkar, S.N., Sawant, V.A., Shende, V.S., Dimble, S.K. (2010):Nanoclay drug delivery System. International Journal of Pharmaceutical Sci-ences and Nanotechnology Volume 3, Issue 2, July - SeptemberTabata, Y., Murakami, Y. and Ikada, Y. (1997): "Photodynamic effect ofpolyethylene glycol-modified fullerene on tumour", Jap J Cancer Res, vol. 88,no. 11, pp. 1108-1116.Takeara, H., Fujiwara, M., Arikawa, M., Diener, M.D., Alford, J.M. (2004):Experimental study of industrial scale fullerene production by combustionsynthesis. Carbon 43 (2), 311–319.Takenaka, S., Karg, E., Roth, C., Schulz, H., Ziesenis, A., Heinzmann, U.,Schramel, P., and Heyder, J. (2001): "Pulmonary and systemic distribution ofinhaled ultrafine silver particles in rats", Environ.Health Perspect., vol. 109Suppl 4, pp. 547-551.
126
Taylor (2008):http://www.nanotechproject.org/process/assets/files/6704/taylor_gma_pen_packaging1.pdfTerrones H., Terrones, M. (2003): The carbon nanocosmos: novel materialsfor the twenty-first century. Filosiphical transaction of the Royal Society, Vol.361, p. 2789-2806.Theogaraj, E., Riley, S., Hughes, L., Maier, M. and Kirkland, D. (2007): "Aninvestigation of the photo-clastogenic potential of ultrafine titanium dioxideparticles", Mut Res, vol. 634, no. 1-2, pp. 205-219.Totsuka Y, Higuchi T, Imai T, Nishikawa A, Nohmi T, Kato T, Masuda S,Kinae N, Hiyoshi K, Ogo S, Kawanishi M, Yagi T, Ichinose T, Fukumori N,Watanabe M, Sugimura T, Wakabayashi K. (2009): Genotoxicity ofnano/microparticles inin vitromicronuclei,in vivocomet and mutation assaysystems. Part Fibre Toxicol. 2009 Sep 3;6:23.Tratnyek and Johnson (2006): Nanotechnologies for environmental cleanup.NanoToday 1(2) 44-48.Trop, M., Novak, M., Rodl, S., Hellbom, B., Kroell, W., Goessler, W.(2006): “Silver coated dressing Acticoat caused raised liver enzymes and ar-gyria-like symptoms in burn patient“, J Trauma, vol. 60, no. 3, pp. 648–652.Tsuchiya, T., Oguri, I., Yamakoshi, Y.N. and Miyata, N. (1996): "Novelharmful effects of [60]fullerene on mouse embryos in vitro and in vivo", FEBSLetters, vol. 393, no. 1, pp. 139-145.Van Hoecke K, Quik JT, Mankiewicz-Boczek J, De Schamphelaere KA, El-saesser A, Van der Meeren P, Barnes C, McKerr G, Howard CV, Van deMeent D, Rydzyński K, Dawson KA, Salvati A, Lesniak A, Lynch I,Silversmit G, De Samber B, Vincze L, Janssen CR. (2009): Environ SciTechnol., 43(12):4537-46.Velzeboer, I., Hendriks, A.J., Ragas, A.M.J. and Van de Meent, D. (2008):"Aquatic ecotoxicity tests of some nanomaterials", Environmental Toxicologyand Chemistry, vol. 27, no. 9, pp. 1942-1947.Wadhera, A., and Fung, M. (2005): Systemic argyria associated with inges-tion of colloidal silver. Dermatology Online Journal, 11(1): 12.Wakefield G., Wu X., Gardener M., Park B. and Anderson S. (2008): Envi-roxTM fuel-borne catalyst: Devel-oping and launching a nano-fuel additive,Technology Analysis & Strategic Management, Vol. 20, No. 1, pp. 127-136Wang J., Zhang X., Chen Y., Sommerfield M. and Hu, Q. (2008): "Toxicityassessment of manufactured nanomaterials using the unicellular green algaChlamydomonas reinhardtii.", Chemosphere, vol. 73, no. 7.Wang, J., Zhou, G., Chen, C., Yu, H., Wang, T., Ma, Y., Jia, G., Gao, Y., Li,B., Sun, J., Li, Y., Jiao, F., Zhao, Y. and Chai, Z. (2007): "Acute toxicity andbiodistribution of different sized titanium dioxide particles in mice after oraladministration", Toxicology Letters, vol. 168, no. 2, pp. 176-185.Wang, J., Chen, C., Liu, Y., Jiao, F., Li, W., Lao, F., Li, Y., Li, B., Ge, C.,Zhou, G., Gao, Y., Zhao, Y. and Chai, Z. (2008a): "Potential neurological
127
lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutilecrystal phases", Toxicology Letters, vol. 183, no. 1-3, pp. 72-80.Wang, J., Liu, Y., Jiao, F., Lao, F., Li, W., Gu, Y., Li, Y., Ge, C., Zhou, G.,Li, B., Zhao, Y., Chai, Z. and Chen, C. (2008b): "Time-dependent transloca-tion and potential impairment on central nervous system by intranasally in-stilled TiO2 nanoparticles", Toxicol, vol. 254, no. 1-2, pp. 82-90.Warheit, D. B., Brock, W. J., Lee, K. P., Webb, T. R. and Reed, K. L.(2005): "Comparative pulmonary toxicity inhalation and instillation studieswith different TiO2 particle formulations: impact of surface treatments onparticle toxicity" Toxicol Sci, vol. 88, no.2, pp. 514-524.Warheit, D. B., Webb, T. R., Reed, K. L., Frerichs, S. and Sayes, C. M.(2007): "Pulmonary toxicity study in rats with three forms of ultrafine-TiO2particles: differential responses related to surface properties. Toxicol, vol. 230,no.1, pp. 90-104.Wiesner, Mark R. (2005): “Science: Toward a green nanotechnology,” DailyTimes, Monday, August 8, 2005,http://www.dailytimes.com.pk/default.asp?page=story_8-8-2005_pg6_7Wijnhoven, S.W.P., W.J.G.M. Peijnenburg, C.A. Herberts, W.I. Hagens,A.G., Oomen, E.H.W. Heugens, B. Roszek, J. Bisschops, I. Gosens, D. VanDe Meent, S. Dekkers, W.H. De Jong, M. van Zijverden, A.J.A.M. Sips, andR.E. Geertsma. (2009): Nano-silver: A review of available data and knowl-edge gaps in human and environmental risk assessment. Nanotoxicology,3:109–138.Winter M, Beer HD, Hornung V, Kramer U, Schins RPF, Forster I. (2010):Activation of the inflammasome by amorphous silica and TiO2 nanoparticlesin murine dendritic cells. Nanotoxicology, Early Online, DOI:10.3109/17435390.2010.506957.Woodrow Wilson Inventory (2011): Project of Emerging Nanotechnologies atthe Woodrow Wilson International Center for Scholars (2011). Nanotechnol-ogy Consumer Products Inventory.http://www.nanotechproject.org/inventories/consumer/analysis_draft/(visited 22 March 2011)Zhu X, Zhu L, Lang Y, Chen Y. (2008): Oxidative stress and growth inhibi-tion in the freshwater fish Carassius auratus induced by chronic exposure tosublethal fullerene aggregates. Environ Toxicol Chem. 2008 Sep;27(9):1979-85.Zhu S, Oberdörster E, Haasch ML.(2006): Toxicity of an engineerednanoparticle (fullerene, C60) in two aquatic species, Daphnia and fatheadminnow. Mar Environ Res. 2006 Jul;62 Suppl:S5-9. Epub 2006 Apr 22.Zhu, X.S., Chang, Y. & Chen, Y.S. (2010): "Toxicity and bioaccumulation ofTiO2 nanoparticle aggregates in Daphnia magna", Chemosphere, vol. 78, no.3, pp. V-215.Zhu, X.S., Zhu, L., Duan, Z.H., Qi, R.Q., Li, Y. and Lang, Y.P. (2008):"Comparative toxicity of several metal oxide nanoparticle aqueous suspen-sions to Zebrafish (Danio rerio) early developmental stage", Journal of Envi-
128
ronmental Science and Health Part A-Toxic/hazardous Substances and Envi-ronmental Engineering, vol. 43, no. 3, pp. 278-284.Yamago, S., Tokuyama, H., Nakamura, E., Kikuchi, K., Kananishi, S., Sueki,K., Nakahara, H., Enomoto, S. and Ambe F. (1995): "In vivo biological be-haviour of a water-miscible fullerene: 14C labelling, absorption, distribution,excretion and acute toxicity", Chem Biol, vol. 2, no.6, pp. 385-389.Yin, J.J., Lao, F., Meng, J., Fu, P.P., Zhao, Y., Xing, G., Gao, X., Sun, B.,Wang, P.C., Chen, C. and Liang, X.J. (2008): "Inhibition of tumor growth byendohedral metallofullerenol nanoparticles optimized as reactive oxygen spe-cies scavenger", Mol Pharmacol, vol. 74, no. 4, pp. 1132-1140.Yong S Kim1,7, Moon Y Song1, Jung D Park2, Kyung S Song1, Hyeon RRyu1, Yong H Chung3, Hee K Chang4, Ji H Lee8, Kyung H Oh5, Bruce JKelman6, In K Hwang7 and Il J Yu1,8 (2010): Subchronic oral toxicity ofsilver nanoparticles. Particle and Fibre Toxicology 7:20.http://www.particleandfibretoxicology.com/content/7/1/20Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI,Nel AE. (2008): Comparison of the mechanism of toxicity of zinc oxide andcerium oxide nanoparticles based on dissolution and oxidative stress proper-ties. ACS Nano. 2008 Oct 28;2(10):2121-34.Xia XR, Monteiro-Riviere NA, Riviere JE. (2010): Skin penetration and ki-netics of pristine fullerenes (C60) topically exposed in industrial organic sol-vents. Toxicol Appl Pharmacol. 2010 Jan 1;242(1):29-37. Epub 2009 Sep 28.Yeo, M.K. and Kang, M. (2008): "Effects of nanometer sized silver materialson biological toxicity during zebrafish embryogenesis", Bulletin of the KoreanChemical Society, vol. 29, no. 6, pp. 1179-1184.
129
130
Annex 1
Annex 1
Nano terminology and ac-ronyms
NanoterminologyThe terms explained below are primarily included to support the reader ofthis report. Several of these terms are currently under discussion and may beadjustded as part of the process of developing regulations and standards in thenanotechnology field.Agglomerate:Collection of loosely bound particles or aggregates or
mixtures of the two where the resulting external surfacearea is similar to the sum of the surface areas of the indi-vidual components. (Particles are held together by rela-tively weak forces, including Van der Waals forces, elec-trostatic forces and surface tension.)Particle comprising strongly bonded or fused particleswhere the resulting external surface area may be signifi-cantly smaller than the sum of calculated surface areas ofthe individual components. (The forces holding an ag-gregate together are strong forces, e.g. covalent bonds.)A covering that is applied to the surface of an object toalter or improve surface properties.Transforming a chemical compound into another com-pound (derivate) of similar structure.Addition of controlled impurities to a semiconductormaterial to change its electrical properties.Addition of functional groups to the surface of the mate-rial in order to achieve specific surface properties.Possessing pores with at least one dimension between 2nm to 50 nm.
Aggregate:
Coating:Derivatisation:Doping:Functionalisation:Mesoporous:
131
Nanomaterial :Nanoobject:Nanoparticles:
11
Material having one or more external dimensions in thenanoscale or which is nanostructured.Discrete piece of material with one or more external di-mensions in the nanoscale.Nano-object with all three external dimensions in thenanoscale. (If the lengths of the longest and the shortestaxes of the nano-object differ significantly (typically bymore than three times) the terms nanorod or nanoplateare intended to be used instead of the term nanoparti-cle.)Size range from approximately 1 nm to 100 nm.Possessing a structure comprising contiguous elementswith one or more dimensions in the nanoscale but ex-cluding any primary atomic or molecular structure.
Nanoscale:Nanostructured:
AcronymsADMEBALBCFCACHL/ILCMC-NaCNTDNADNELEC50FCM11
Absorption Distribution Metabolism ExcretionBronchoAlveolar LavageBioconcentration FactorChromosomal AberrationsChinese Hamster Lung / InterleukinMethylcellulose sodiumCarbon NanoTubesDeoxyribonukleinsyreDerived no Effect levelEffective Concentration 50%Food Contact Materials
A formally accepted definition of the term "nanomaterial"is not yet accepted. TheEuropean Commission has proposed the following definition to be used as the over-arching, broadly applicable reference term for any European Union communicationor legislation addressing nanomaterials, but this may to subject to change in the fu-ture:Nanomaterial: means a material that meets at least one of the following criteria:– consists of particles, with one or more external dimensions in the size range 1 nm- 100 nm for more than 1 % of their number size distribution;– has internal or surface structures in one or more dimensions in the size range 1nm – 100 nm;– has a specific surface area by volume greater than 60 m2/cm3, excluding materi-als consisting of particles with a size lower than 1 nm.
132
Annex 1FPGINELLC50LD50LDHLOAECLOECMMCMNNOAELNOAECNOECNOAELNOELNPNSPPECPNECREACHROSSNPTEMFormamido-pyrimidine-DNA-glycosylaseIndicative Effect levelLethal Concentration 50%Lethal Dose 50%Lactate DeHydrogenaseLowest Observed Adverse Effect ConcentrationLowest Observed Effect ConcentrationMitomycin CMicronucleusNo Observed Adverse Effect LevelNo Observed Adverse Effect ConcentrationNo Observed Effect ConcentrationNo Observed Adverse Effect LevelNo Observed Effect levelNanoparticlesNano-Sized ParticlePredicted Environmental ConcentrationPredicted No-Effect ConcentrationRegistration, Evaluation, Authorisation of ChemicalsReactive Oxygen SpeciesSilica NanoParticleTransmission Electron Microscopy
133
134
Annex 2
Companies that commer-cialise nanotechnologyand / or nano materials inDenmark
Companies that commercialize nanotechnology and/or nanomaterialsin DenmarkRecently, the Nanowerk published an online Company & Labs directory with4,196 links to labs, associations, networks and companies. This directory in-cludes only companies and labs that work with and/or commercialisenanotechnology and/or nanomaterials and does not include entities that onlyhave “nano” in their name.According to Nanowerk there are 18 companies in Denmark involved in vari-ous fields of technology commercially.1.AQUAporin:The company is committed to revolutionizing water pu-rification through bionanotechnology with the development of a highlywater-selective membrane.2.Cantion:Provides nanomechanical products such as nanonanofluidicdispensers and nanomechanical cantilevers for label free detection andanalysis of molecules.3.Capres - Copenhagen Applied Research:CAPRES develops newtechnology for direct nano- and micro-scale electrical characterizationof materials.4.DME Danish Micro Engineering:Develops and manufactures Scan-ning Probe Microscopes.5.Haldor Topsoe:Topsoe specialises in the production of heterogene-ous catalysts and the design of process plants based on catalytic proc-esses. Focus areas include the fertiliser industry, the chemical and pet-rochemical industries, and the energy sector (refineries and powerplants). Topsoe has for many years dedicated large research efforts tonanoscience and nanotechnology. The efforts range from the devel-opment of novel preparation methods to theoretical and experimentalinvestigations.6.Image Metrology:Nanoscale image processing software and 3D visu-alization.7.InMold Biosystems:InMold Biosystems proprietary technology offersa radical new approach to increase the functionality of plasticware.Biomolecules, like proteins, may be transferred to flat or structuredsurfaces in a pattern with nanometer sized details by micro contact
135
printing.8.LiPlasome Pharma:The combination of a protected blood transport-ing nanocarrier system and a tumor specific activation technologymakes LiPlasome Pharma very competitive in a commercially attrac-tive and dynamic anticancer market, where drug delivery systems willgain increasing importance over the coming years.9.NanoCover:NanoCover specialises in the production, distribution,sales and development of NanoCover, a series of surface treatmentproducts. NanoCover is developed and produced using the newestand most advanced nanotechnologies.10.Nanofiber:Develops protocols for growth and transfer of morphologi-cally and optically controlled organic nanofibers.11.Nanon:Specializes in the nanoscale manipulation of 'difficult' poly-mers, such as silicone, with the goal of optimizing performance - with-out compromising the properties of the base material.12.NanoWorld I/S:NanoWorld I/S specializes on research, test and dis-tribution of nano-based surface sealants. The company offers a widerange of nano sealing products for private and professional use.13.NIL Technologies:The company sells stamps for nanoimprint lithog-raphy (NIL), provides imprint service, production by NIL, consul-tancy and enters into joint development of novel applications benefit-ing from nano-scale structures.14.Noliac:Designs, develops and manufactures the total range of piezo-electric products - from powders to mono- and multilayer componentsand all the way to finished plug-and-play applications.15. Proxeon:Proxeon develops state-of-the-art solutions to solve many ofthe scientific and technical challenges that are faced by those workingin the field of proteomics. The company's core competencies are inthe areas of mass spectrometry, protein analysis, hardware and soft-ware design for proteome analysis.16.QuantumWise:QuantumWise produces software for atomic-scalemodeling of nanoelectronic devices, wrapped in a platform with agraphical user interface and a scripting language interface. The com-pany is unique in offering a commercial code that can do transportcalculations using first-principles methods, and at the same time theback-end provides state-of-the-art simulation capacity for large-scalesystems, allowing for systems with over 1,000 atoms to be simulatedon a single workstation.17.SCF Technologies:SCF Technologies A/S develops and commercial-izes technologies, such as nanostructure materials, that are used to re-fine products, enhance processes and produce intelligent materials.18.SunFlake:SunFlake is developing a new generation of solar cells basedon a novel shape of semiconductor nanostructures (NanoFlakes). Thenanostructures can eliminate the need for a lattice-matched substrateas well as the need for a clean solar grade substrate (Nanowerk 2010).
136