Udvalget for Fødevarer, Landbrug og Fiskeri 2010-11 (1. samling)
FLF Alm.del Bilag 133
Offentligt
TESTBIOTECHTestbiotech e. V.Institute for IndependentImpact Assessmentin Biotechnology
Risk ReloadedRisk analysis of genetically engineered plantswithin the European UnionA report by Testbiotech e.V.Institute for Independent Impact Assessment inBiotechnologywww.testbiotech.orgOctober 2009Christoph Then, Christof PotthofEditing: Andrea Reiche
Risk Reloaded:Risk analysis of genetically engineered plantswithin the European UnionA report byTestbiotech e.V.Institute for Independent Impact Assessmentin Biotechnologywww.testbiotech.org
ImprintTestbiotech e.V.Frohschammerstr. 1480807 MünchenTel.: +49 (0) 89 358 992 76Fax: +49 (0) 89 359 66 22[email protected]www.testbiotech.orgExecutive Director: Dr. Christoph ThenOctober 2009
Contents| Risk Reloaded | 3
Contents0406070709101112121214151516161719192020212222232325262729313133343643SummaryIntroduction1. Concepts of risk assessment1.1 The controversy of feeding trials1.2 General prerequisites for risk assessment1.3 Assumptions and hypotheses underlying EU risk assessment1.4 Substantially equivalent or different?1.4.1 Networks of regulation instead of isolating genes1.4.2 Optimising existing potentials or reprogramming by technical intervention?1.4.3 Collaterals1.4.4 What is biologically significant?2. Actual standards for risk assessment and monitoring in the EU2.1 Feeding trialsTable 1: Selected feeding studies as accepted by EFSA2.2 Ecological risk assessment2.3 Monitoring3. New doubts about safety3.1Plants producing insecticidesFig. 1: some theories for mode of action of Bt -toxin (Cry1Ab)3.2 Herbicide-tolerant plants3.3 ‘Stacked events’ and interactions between transgenic plants3.4 General risks from genetically engineered plants3.4.1 Risks to immune system3.4.2 How the environment impacts transgenic plants and the introduction of crash testsTable 2: factors impacting Bt content in MON810 maize (source: Then & Lorch 2008)4. How independent are public research and the authorities?4.1 An illustration: feeding trials with cows and the dairy industry4.2 Special agencies’ networkInterview by Christof Potthof with bee researcher Hans-Hinrich Kaatz,University of Halle5. Political responsibilitiesFig. 2 : Risk assessment policies6. Some recommendationsTable 3: Step by step procedure for early stages of integrated risk analysis of geneticallyengineered plantsReferencesConcluding remarks
4 | Risk Reloaded |Summary
SummaryThis is a report on the risk assessment procedure for genetically engineered plants in the EU. It revealssubstantial flaws and loopholes in the procedure and practice of the institutions concerned. Many ofthe flaws have their origin in the European Food Safety Authority’s (EFSA) own main concept of riskassessment. This is essentially based upon guidelines that were developed by the OECD as early as1993 on the assumption that the risks posed by genetically engineered plants are basically the sameas those posed by conventional plants. This approach has admittedly been revised several times since1993 but has in essence remained unchanged.The report shows that the current guidelines are inadequate for sound risk assessment. New findingsin genome research have in recent years transformed ideas of gene regulation and gene function. It hasbecome evident that invasive intervention in genetic makeup and the transfer of isolated genes cannotbe equated with natural mechanisms of heredity and gene regulation. The basic difference betweenconventional cultivation and genetic engineering of plants is becoming more and more distinct in thelight of current genome research. Experience gained from cultivating conventional plants cannot – oronly to a very limited extent - be applied to genetically engineered plants.Even in conventional cultivation there are many changes in the genome but these do not break throughthe natural system of gene regulation. In contrast a new metabolism is forced upon genetically engi-neered plants. In fact the regularly observed changes in the activity of plant genes in this process arenot an expression of natural gene regulation but an indication of disruption. These transgenic plants1are technically manipulated products and as such much be assessed unconditionally for constructionalflaws, quality defects and risks.The outdated basic concept of the OECD from 1993 and the subsequent concepts developed for riskassessment of genetically engineered plants (FAO/WHO, 2000; Codex Alimentarius, 2003; EFSA, 2006)mean that the safety, predictability and controllability of genetically engineered plants are not exam-ined in detail within the framework of approval procedure.Irradiated food, pesticides, chemicals and medicines are all unconditionally tested for possible risks. Inorder to thoroughly test genetically engineered plants, however, there first of all has to be some proofthat there may be a risk. Genetically engineered (GE) plants are deemed to be safe as long as no proofto the contrary has been produced. This means that GE plants are tested much more superficially thanirradiated food, pesticides, chemicals and medicines.Overall the concept as defined by the EFSA (2006, 2007a, 2007b) does not meet the requirements of theEU for comprehensive testing. It replaces actual risk testing by a system of presupposed assumptionsbased upon conclusions that are hardly verifiable.In this report the authors give an overview of the reasons for recent doubts about the safety of geneti-cally engineered plants and present different examples which show the inconsistencies and failuresin EFSA’s risk assessment. One of the examples used to expose the lack of essential requirements forwell-founded risk assessment is MON 810 maize which produces the Bt insecticide.It is also extremely problematic that more and more cases are being documented showing that indepen-
1
In this report no distinction is made between transgenic and so called cisgenic plants, because both are the result oftransferring isolated gene sequences
Summary| Risk Reloaded | 5
dent risk research is being hampered. In many cases it is not even possible to access necessary testingmaterials. Even the publication of findings is being obstructed. All in all the influence of industrialinterests in research and the presentation of findings have reached alarming proportions.Against the backdrop of various political discussions on the further development of testing standardsin the EU, the authors make concrete suggestions on how testing systems can be improved to generatemore data on the quality and safety of genetically engineered plants. They advocate more extensivetesting on the compounds and genetic stability of GE plants before they are released into the field andcompanies can apply for market authorisation.The plants should be subjected to a suitable level of exposure in specific “crash tests” to test theirreaction to changing and extreme environmental conditions. Before field release and in order to collectmore data on potential risk they should be tested (i.a. with different microorganisms) in a containedsystem to detect any interaction between the plants and a simulated environment. The authors suggestthat these tests be introduced in the course of introducing improved step by step, case by case testswhich have clearly defined test criteria for genetically engineered plants; with concomitant strongercollaboration between the authorities of member states and EU authorities and a higher consideration ofethical and socio-economic factors. The documentation of relevant information shall become a precondi-tion for EU authorisation procedures.Society, politics and approval boards should no longer close their eyes to the fact that agro-genetechnology uses methods that are largely outdated and whose risk potential is higher than originallythought. It is not the fear of new products that make a critical appraisal of agro-gene technology neces-sary, but rather the fact that its scientific principles have been called more and more into question bynew findings.
6 | Risk Reloaded |Introduction
IntroductionThe New York Times in 2007 featured an article about recent results in genome research which putin question long established views about genes and their regulation.2The international Encode projectshowed that the mechanisms in gene regulation are much more complex than had been thought so far(Encode, 2007). In the words of the New York Times:“The scientists who invented recombinant DNA in 1973 built their innovation on this mechanistic, “onegene, one protein” principle. Because donor genes could be associated with specific functions, with dis-creet properties and clear boundaries, scientists then believed that a gene from any organism could fitneatly and predictably into a larger design - one that products and companies could be built around,and that could be protected by intellectual-property laws. This presumption, now disputed, is whatone molecular biologist calls “the industrial gene”. “The industrial gene is one that can be defined,owned, tracked, proven acceptably safe, proven to have uniform effect, sold and recalled,” said JackHeinemann ...”The Encode project once again showed what had already been broadly discussed amongst experts in2001. At this time the first analysis of the human genome was presented, showing that humans onlyhave about 20,000 instead of about 100,000 genes, as had previously been thought. This remarkablylow number of human genes is in striking contrast to their task to code for hundreds of thousands ofproteins in the human body. Craig Venter, who played a decisive role in the human genome project,made an astonishing comment in Science (Venter et al. 2001):“The modest number of human genes means that we must look elsewhere for the mechanisms thatgenerate the complexities inherent in human development and the sophisticated signaling systemsthat maintain homoestasis.”These findings of genome research gave birth to something which is called by many experts the eraof postgenomics. It is no longer the analysis of specific DNA sequences that catches the most scientificattraction, but the mechanisms of its complex regulation. This new focus of research is also widely ac-cepted in plant genomics (see for example Clark et al., 2007).The TestBioTech report presented here starts with the question of the extent to which this break-through in molecular biology also impacts genetic engineering in plants and their risk assessment.Several concepts for risk analysis are discussed against this background. Special attention is given tothe practice of the European Union.
2
Caruso, Denise, „A Challenge to Gene Theory, a Tougher Look at Biotech“, New York Times, 1. Juli 2007,www.nytimes.com
Concepts of risk assessment| Risk Reloaded | 7
Concepts of risk assessmentIn the year 2000 it was reported that some experts had succeeded in genetically manipulating ricekernels to produce beta-carotene in their endosperm (Ye et al., 2000). The carotenoid can be used by thehuman body as a source for the production of essential Vitamin A. Because the kernels had a yellowcolour (caused by an unintended effect), they were very soon called “Golden Rice” (for review see Then,2009a). In spring 2009 it got noticed that trials with school children were conducted without the poten-tial health effects of the transgenic rice being comprehensively tested beforehand.3Experts defendingthe project rejected any criticism by claiming that the risk could be rated as being minor.4The websiteof the Golden Rice Consortium even featured a statement saying the risks to school children would becomparable to those of eating a small carrot:“The experiments were no more dangerous than feeding the children a small carrot since the levels ofbeta-carotene and related compounds in Golden Rice are similar.”5Experts at Tufts University, USA, and elsewhere have a different opinion. They published the first re-sults of testing the genetically engineered rice in adult volunteers in the US, and explicitly mention thatclinical trials cannot be avoided if the safety of the product is to be investigated (Tang et al., 2009).The debate about this project, which has been going on for years, highlights the general controversyabout risk assessment standards. Even more than ten years after the first commercial cultivation ofgenetically engineered plants (such as soy with herbicide tolerance or maize producing insecticides)there is no generally agreed perception about the risks inherited by those plants nor about the way toconduct proper risk assessment. There have been efforts to reach international consensus in papersdrawn up by the FAO and WHO (FAO/WHO 2000) and various documents prepared by the OECD6, aswell as within the Codex Alimentarius (2003), which the European Food Safety Authority (EFSA) alsouses as a basis. But as explained below these concepts cannot be seen as being a solution to the exist-ing problems. These minimal standards are not in line with more recent scientific findings in molecularbiology and not in accordance with the EU’s legal requirements.The main principle behind these international standards is the concept of ‘substantial equivalence’developed by the OECD as long ago as 1993. This concept was harshly criticised by many stakeholdersand experts but is still seen as the starting point for risk assessment (FAO/WHO 2000, Codex Alimen-tarius 2003). The notion works in practice as a hypothesis or a general assumption which is madebefore any real risk assessment takes place, thereby influencing the outcome of risk assessment in asignificant way.
1.
The controversy about feeding trialsAn example of the controversies caused by this hypothetical practice is the question of whether feed-ing trials are necessary to test the safety of genetically engineered food, something already mentionedin connection with Golden Rice. The EFSA in 2007 conducted a specific report on this issue, referringto consensus documents from the FAO, WHO, OECD and Codex Alimentarius, as mentioned above. TheEFSA report explains why the authority does not in general perceive feeding trials with geneticallyengineered plants (or derived food and feed) as being necessary. The EFSA (2007a) proposes a standardfor risk assessment for genetically engineered plants (and derived food and feed) that is essentially dif-ferent from those being used for radiated food, pesticides, chemicals or pharmaceuticals.
1.1
3456
http://www.goldenrice.org/Content2-How/how3_biosafety.htmlhttp://www.dailymail.co.uk/news/worldnews/article-1147635/British-scientists-condemn-using-children-GM-food-trials-unacceptable.htmlhttp://www.goldenrice.org/PDFs/Daily_Mail_Letter_Feb_2009.pdfOECD Consensus Documents for the work on the Safety of Novel Foods and Feeds, OECD.http://www.oecd.org/document/9/0,2340,en_2649_34391_1812041_1_1_1_37437,00.html
8 | Risk Reloaded |Concepts of risk assessment
To prove the safety of radiated food, for example, feeding trials were conducted on mice, rats, dogs,monkeys and even humans (WHO, 1999). Feeding trials were performed over a duration of severalyears to investigate growth, carcinogenicity and reproductiveness:“The safety of high-dose irradiated foods has been evaluated in many feeding studies conducted overthe past four decades that have involved a variety of laboratory diets and food components given tohumans and a broad cross-section of animal species, including rats, mice, dogs, quails, hamsters,chickens, pigs and monkeys. These investigations, which have included subacute, chronic, reproduc-tive, multigeneration and carcinogenicity studies, have been conducted under a variety of experimen-tal protocols and have covered a range of doses.”These kinds of feeding trials can be seen as being not necessary, and even immoral if the introductionof radiated food is seen as superfluous. But should they also be regarded as being completely uselessfrom a scientific point of view?By rejecting feeding trials using whole transgenic plants as suggested by the EFSA (2007a), one hasto face a specific problem: risks emerging from products consisting of complex mixtures, includingvarious different compounds, can hardly be assessed by just analysing some of their isolated com-ponents. In the case of genetically engineered maize, rice or soy, some of the plants at the same timetolerate herbicides (and are likely to have some residues from its application) and produce insecticides.These so-called stacked events (plants inheriting a combination of more than one transgenic trait) arecultivated in the US and some of them are also allowed for import into the EU. Cross activities betweenvarious compounds in the plants during their cultivation might produce results which can hardly bepredicted from analyses of their single components.These problems are moreover also relevant for transgenic plants that only inherit a single transgenictrait. Unintended effects can also occur in these plants with unpredictable impacts to human healthand the environment. As Batista et al (2008) show, for example, the activity of many genes can be al-tered by inserting single gene sequences (see below). Because of these unintended effects experts suchas Spök et al (2004) and Seralini et al (2009) explicitly urge feeding trials using the whole plants andnot only single compounds, to get a better understanding of the associated risks. As Spök et al (2004)explain:“Testing should be extended to include whole-plant/whole-food testing in both toxicity and allergenic-ity studies in order to more reliably detect unintended and detrimental effects of genetic modification.”These arguments are not followed by the EFSA. The European Food Safety Authority only suggestsmore detailed investigations in connection with products such as the Golden Rice (for which marketauthorisation has so far not been applied). According to the EFSA the metabolism of these plants can beregarded as being changed on several levels, so feeding trials with whole plants should be performedin order to avoid negative health effects. By arguing this way the EFSA (2007a) contradicts the positionof the Golden Rice team. But it at the same time assumes that the transgenic plants where approval ofcommercialisation is currently being applied for should be seen as harbouring only minor risks. TheEFSA does not consider feeding trials to be necessary even in the case of stacked events (EFSA 2007a).The EFSA generally assumes that risks to human health can be deduced from the analysis of singlecompounds at least in the case of transgenic plants tolerant of herbicides or producing insecticides. Theauthority argues that the transfer of genes would change the plants only in relation to certain charac-teristics. Besides that, genetically manipulated plants can be seen as equivalent to plants derived fromconventional breeding (EFSA 2007a):“The current generation of GM plants cultivated for commercial purposes has been modified throughthe introduction of one or a few genes coding for herbicide tolerance, insect resistance or a combina-tion of these traits. In these plants the genetic insert leads to the production of a gene product, whichdoes not interfere with the overall metabolism of the plant cell, and does not alter the composition ofthe GM plant except for the introduced trait.”
Concepts of risk assessment| Risk Reloaded | 9
Where these plants are concerned it is assumed that unpredictable risks are not likely to arise, so thatfeeding trials with the whole plants are not necessary. If these transgenic plants do not show clearsigns of unintended effects it is enough to screen for specific compounds, more detailed investigating ofhealth risks not being necessary.While risks to human health of products such as radiated food, pesticides and pharmaceuticals haveto be investigated to prove their safety without anything being presumed, in the case of geneticallyengineered plants the risks first have to be proven before detailed investigations are made.
General prerequisites for risk assessmentAccording to the regulations of the European Union (Regulation 178/2002 and Directive 2001/18) andits underlying basis as elaborated in the White Book of the European Commission (Commission ofEuropean Communities, 2000), a high level of protection of the environment and consumers are theoverarching goals of the EU’s policy. In the case of uncertainties the precautionary principle shall pre-vail. In comparison the economical expectations associated with the commercialisation of geneticallyengineered plants have to be seen as being of minor importance.Against this background it has to be acknowledged that risk assessment of genetically engineeredplants shows a high level of complexity, since ecological impact, agronomic performance, human healthaspects and questions regarding molecular biology and toxicology have to be taken into account. Somerelevant issues are:1. Molecular biology.- The place of insertion of new genes into the plants’ genome is not the result of atargeted process, but is more or less based on chance. Methods like the ‘particle gun’, or more or lessscattered shot technologies, are still being used. This can lead to distortions of the plants’ genome at theplace of insertion. Further, the biological activity of newly inserted gene sequences has to be enforcedartificially (see for example Diehn et al, 1996). The plants’ own gene regulation has to be knocked out(partially) to avoid silencing the additional gene constructs. The activity of various other genes can bealtered by the genetic manipulation. This effect can be observed with gene sequences that are locatedat a far distance in the plants’ genome as well as with gene sequences that are in the direct neighbour-hood of the newly inserted genes. In Monsanto’s genetically engineered soybeans, for example, Rang etal (2005) showed that the neighbouring gene sequences of the plant get fused with the artificial geneconstruct and show some unexpected activity. Similar results were described in genetically engineeredmaize by Rosati et al (2008). These unintended biological interferences can have various effects at thelevel of the genome, cell metabolism and or the whole organism. In some cases this interference hasbeen overlooked for several years even after market authorisation.2. Toxicology and health impacts. - The range of intended compounds that are produced in the trans-genic plants covers substances meant to protect human health up to toxic proteins meant to defeat pestinsects. In addition one has to take into account unintended components caused by occasional interfer-ence of the plants’ metabolism.3. Environmental impact. -The relevant risks are not of a steadily fixed nature, they involve dynamicprocesses involving factors like the growth of the plants and various environmental influences. Insecti-cides in genetically engineered cotton and maize, for example, are influenced by several environmentalconditions (Then&Lorch, 2008a). Exposed to external stress factors, genetically engineered plants canalso exhibit unexpected effects not noticed before (Matthews et al, 2005).4. Unintended gene transfer. - Gene sequences enabling resistance against antibiotics can be transferredto hazardous microorganisms, at least in theory. Debates and even minority votes within the EFSA’s
1.2
10| Risk Reloaded |Concepts of risk assessment
GMO panel show that this risk is still seen as controversial after many years.7The potential healtheffects of inserted gene sequences (gene constructs) if used in food and feed (as for example argued inCotter&Mueller, 2009, Myhre et al., 2006) are another point of discussion. Special cause for concern isthe fact that the spread of the artificial gene constructs via pollen and other escape routes cannot beprevented.All in all one has to deal with a complex matter of ecological, biological and health related issues whichis dependent on a broad range of additional external factors which are not fully understood in alldetails even after many years of research. Traavik (2008) describes the situation:“The dynamic and interconnected regulation of the genome is now slowly being revealed. The genomedoes not function in a constant, stable and linear fashion, but is instructed by and fine-tunes itsactivities according to networks of signals received from the external ecosystem and the internalenvironment of the organism. The genomic signal pathways may be modified by ecosystem variationas well as by physiological changes in the organism. Thus, the chromatin structure, the genome, theepigenome, the transcriptome, the proteome, the metabolome, and the interactome are interlinked andintertwined in various ways with information transfer in multiple directions.”The recent political debates in the EU show how difficult it really is to develop a system for risk assess-ment that has a sufficient scientific basis. The EU Commission8as well as the members states9haverepeatedly been highly critical of the work of the EFSA’s GMO panel in the last few years.
1.3
Assumptions and hypotheses underlyingEU risk assessmentAccording to the ‘Guidance Document of the Scientific Panel in genetically modified organisms for therisk assessment of genetically modified plants and derived food and feed’, which the EFSA has drawnup as a basis for its risk assessment (EFSA 2006), the underlying principle of its risk assessment is aso-called “comparative approach”, which tries to draw a comparison between the genetically engineeredorganisms and their counterparts produced in conventional breeding. In this approach the EFSA usesthe concept of “familiarity“ for the risk assessment of the cultivation of transgenic plants and “substan-tial equivalence” for the risk assessment of food and feed (EFSA, 2006).The underlying hypothesis of this principle implies that genetically engineered plants are not com-pletely new organisms but similar to plants as used for centuries in conventional agriculture and onlychanged in distinct characteristics. Thus the plant derived from conventional breeding and the trans-genic plant are defined as being substantially equivalent as long as no major differences can be foundby comparing plant compounds and agronomic characteristics:“The concept of familiarity is based on the fact that most GM plants are developed from organismssuch as crop plants, the biology of which is well researched. In a risk assessment it is appropriate todraw on this previous knowledge and experience and to use the non-GM crop as the comparator to theGM crop [...]”and:“The concept of substantial equivalence is based on the idea that an existing organism used as food/feed with a history of safe use, can serve as a comparator when assessing the safety of the geneticallymodified food/feed [...].” (EFSA, 2006)Risk assessment can thus be reduced to selected distinct aspects. But this principle of a “comparativeapproach” can easily provoke wrong interpretations. If - due to insufficient scientific methods - no sub-789http://www.efsa.europa.eu/EFSA/Statm_of_Efsa/gmo_biohaz_st_ej1108_ConsolidatedARG_en.pdf?ssbinary=truehttp://europa.eu/rapid/pressReleasesAction.do?reference=IP/06/498&format=HTML&aged=1&language=EN&guiLanguage=enhttp://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/envir/104553.pdf
Concepts of risk assessment| Risk Reloaded | 11
stantial differences can be found between the transgenic plant and its comparator, the product is likelyto be categorised as being safe. So if for example adequate methods for detailed investigation of theproteome are not available and thus certain differences are overlooked, the product will be categorisedfalsely as being substantially equivalent. It is important here to know that methods especially relevantin the analysis of the proteom and the metabolom have not yet been developed sufficiently to be usedas standard procedures in risk assessment (EFSA 2007 a), despite these methods having been seen asdecisive tools for several years.The underlying hypothesis and assumptions of substantial equivalence and familiarity are not a matterof discussion in the EFSA’s daily work – they are simply seen as a precondition. The authority will asa result classify a product simply as being safe if it does not reveal specific risks after some initial su-perficial investigation. Furthermore the concept as applied by the EFSA leaves substantial room for as-sumptions and interpretation. Whether or not even significant differences between the transgenic plantand its comparator are interpreted as being substantially equivalent or not (see below) can depend onthe point of view of particular experts.A completely different concept of risk assessment would apply if the underlying hypothesis took intoaccount the fact that transgenic plants are derived from a technical process that cannot be compared tomethods used in conventional breeding. The resulting products therefore have to be classified as tech-nical constructs that have no real counterpart in plants derived from conventional breeding. In risk as-sessment genetically engineered plants have to be investigated without any restriction to any relevanttechnical and biological details. One could for example imagine something like a crash test as beingapplied to other technical products to reveal hidden risks and technical deficiencies. Even the EFSAadmits that a completely different approach to risk assessment would be necessary if the hypothesis ofsimilarity and comparability were not the starting point of its assessment:“Where no appropriate comparator can be identified, a comparative safety assessment cannot bemade and a comprehensive safety and nutritional assessment of the GM crop derived food/feed per seshould be carried out. For instance, this would be the case where a trait or traits are introduced withthe intention of modifying the composition of the plant significantly.” (EFSA 2006)Such a comprehensive approach to a risk assessment without the precondition of similarity and com-parability has not been applied by the EFSA in any case so far. In the light of more recent findings inmolecular biology the necessity for comprehensive testing is becoming evident, since it enables betterunderstanding of the potential impacts of the technology used to create transgenic plants.The need to treat genetically engineered plants as organisms that do not have a history of safe usebecause they are created by methods that cannot be compared to any other method of breeding wasraised from the very beginning of this technology. But this was always harshly rejected by the propo-nents of genetic engineering and very soon also rejected by US legislation. As is argued below, it is timeto correct this development.
Substantially equivalent or different?The basic difference between conventional breeding and genetic engineering is becoming more andmore evident because it can be shown that the mechanism for the regulation of the genome is far morecomplex than was estimated some years ago. This reveals the insufficiency of concepts and principlessuch as comparability, equivalence or familiarity.
1.4
12| Risk Reloaded |Concepts of risk assessment
1.4.1
Networks of regulation instead ofisolating genesSince the human genome project was completed, general understanding about gene function andgenome regulation has changed substantially. Before it was quite common to define a gene as a distinctDNA sequence with a fixed function. But since then the pattern of explaining a gene’s mechanism hasbeen getting more and more complicated. Mattick (2003) for example describes genes as “fuzzy tran-scription clusters with multiple products”.The idea of genome regulation has changed drastically not only for humans and mammals (ENCODE2007), but also for plants (see, for example, Clark et al., 2007). All in all the organisation of the genomeis much more defined by networks and (quantitative) synergies of gene clusters than by the functionof single genes. Wentzell et al. (2008) for example describe how networking of genes can influence theplants’ characteristics and also interfere with the environment, with this based on mechanisms thatare far beyond being understood in all their details:“Most phenotypic variation present in natural populations is under polygenic control, largely deter-mined by genetic variation at quantitative trait loci (QTLs). These genetic loci frequently interact withthe environment, development, and each other, yet the importance of these interactions on the under-lying genetic architecture of quantitative traits is not well characterized.”The Syngenta company is using similar expressions in its patent application WO2008087208:“Most phenotypic traits of interest are controlled by more than one genetic locus, each of which typi-cally influences the given trait to a greater or lesser degree (...) Generally, the term “quantitative trait”has been used to describe a phenotype that exhibits continuous variability in expression and is the netresult of multiple genetic loci presumably interacting with each other and/or with the environment.”These complicated and complex models of gene functions are not well suited for transferring isolatedgenes beyond the barriers of species. Predicting the biological role of a DNA sequence that gets isolatedand transferred to another organism looks like a matter of major uncertainty, since the biologicalfunction of a gene is always defined by its background (or environment). As Pickardt (2002) writes: “Agenetically transferred gene sequence must then be understood as genetic information the context ofwhich has been altered in an uncontrolled way.” Thus this new insight into gene function and genomeregulation also has a substantial impact on the development of hypotheses on the risk assessment ofthe transfer of isolated genes (see also Greenpeace, 2005).
1.4.2
Optimising existing potentials orreprogramming by technical intervention?To get a closer idea of the differences between transgenic methods and conventional breeding it mightbe helpful to take a closer look onto the technical procedures used to create a plant with an artificialgene insert. Diehn et al. (1996) describe the creation of plants producing insecticides. By introduc-ing gene sequences for so called Bt toxins (insecticides normally produced by the bacteria Bacillusthuringiensis) plants such as maize and cotton were armed to resist pest insects. Before the bacterialgene was successfully expressed in the plants, it was necessary to overcome several technical andbiological hurdles:In a first attempt the DNA was transferred to plants in the full length and structure it occurs in bacte-ria. But the plants showed only a very low content of the toxin.In a next step the gene sequence was shortened, and some non essential parts were cut off. The genesequences showed a higher biological activity but the level of toxin in the plants was not high enoughto kill the pest insects.
Concepts of risk assessment| Risk Reloaded | 13
In a further trial the promotor (derived from cauliflower mosaic virus) was doubled to enhance thebiological activity of the Bt gene. As a result the Bt genes showed a tenfold higher activity.But even this technically derived enhancement was not sufficient for the level of the Bt toxin necessaryto kill the pest insects to be reached. It took some further changes in the structure of the Bt genes tofinally reach the desired result.This example shows that several hurdles have to be overcome in natural existing gene regulation toenable the biological activity of transferred gene sequences. Diehn et al. (1996) are of the opinion thattheir observations reveal basic mechanisms used by plants to protect themselves against the transferof foreign DNA and its products:“The mechanisms that limit the expression of unmodified Bt toxin genes are vital plant mechanismsthat naturally affect endogenous RNA and protein levels.”It is also known from effects called gene silencing that plants try to defend themselves against theinsertion of additional genes. Even if foreign genes get activated successfully they can still be switchedoff by epigenetic effects in the plants (see Finnegan, 1994; review by Moch, 2006).One can thus say in summary that the new genetic information and its expression in the cells have tobe forced into the plants by technical means. The normal mechanisms of gene regulation have to besurrounded or even (partially) knocked out. Invasive methods like this are not used in conventionalcrossings or mutation technologies. The mechanisms of normal gene regulation are not changed by anyother breeding method. If, for example, new genetic information obtained inconventional breeding (forexample caused by mutations) will only be used if it fits with the existing genetic background of theplants (e.g. see Fernandez-Martinez et al. 1997, for general background see Nijhout, 2003).For the risk assessment of genetically engineered plants it is crucial not to deny the basic distinctionbetween methods for breeding and technical construction of genetically engineered plants. Whileconventional breeding uses existing biodiversity and its potentials developed by evolution over a longperiod of time, genetic manipulation tries to enforce a technical program without obeying the rules ofnormal gene regulation.
CollateralsThe changes associated with genetically engineering plants are not restricted to specific regions inthe genome. Experimental investigations with fruit flies show that change in biological activity of onegene can induce changes in the activity of several hundred other genes (Anholt et al., 2003). It is alsoknown from the transfer of genes to plants that this process impacts the genome and cell regulationon several levels (Wilson et al. 2006). There seems to be a common understanding about the fact thatunintended effects in genetically engineered plants can be expected in many cases (see Kuiper et al.,2001), but how to interpret these findings is a matter of dispute. Many changes in the genome andmetabolome have also to be expected in conventional (and mutation) breeding. Several recent investiga-tions compare these changes in transgenic and non transgenic plants. But in assessing its outcome onehas to differentiate between several aspects. Some of those issues shall shortly be discussed here usinga publication by Batista et al. (2008):Batista et al. tried to compare the changes in gene activities in plants which had been subjected tomutation breeding and genetic engineering. In transgenic plants they found 2,318 additional changesin gene activity. The number of genes with a changed activity was reduced in the following generationsbut even in those plants some significant additional changes could be demonstrated.Batista et al. demonstrate that in mutagenisised plants even more changes in gene activity can beobserved. They thus also propose to investigate the risks of products derived from mutated plants. But
1.4.3
14| Risk Reloaded |Concepts of risk assessment
such a straight analogy between genetic manipulation and artificial mutation cannot be made. Mutationbreeding is essentially based on evolutionary mechanisms: plants are permanently subjected to signals(such as UV sunlight) that can induce mutations. Thus the plants are trained to protect their genome;research shows that the number of mutations finally used is very minor.Further, the change of gene activity in the follow up of a radiation cannot be seen as unexpected. It sim-ply shows the plants’ normal reaction (including repair mechanism) to an unspecific, untargeted stressfactor. On the contrary, genetic engineering in plants aims at a specific manipulation in the plants’ ge-nome which cannot be controlled by the plant and uses genetic information that is not familiar with themetabolism of the plant. Thus the observed changes in gene activity of genetically engineered plantshave other causes and so can result in other effects. All in all a multiple change in biological activismof genes is not surprising in mutagenizised seeds. But it is a matter of concern that in transgenic plantsso many genes are affected by the introduction of new genetic information which is believed to bespecific.What is regarded as a normal mechanism in the gene regulation of plants in conventional cultivation(and basically in mutation breeding too) must in the case of direct intervention in the genome viagenetic engineering be interpreted much more as disrupting its gene regulation. In any case the find-ings of Batista et al (2008) show that the assumptions of the EFSA, supposing changes in geneticallyengineered plants to be restricted only to the specific trait inserted, are not based on scientific facts.
1.4.4
What is biologically significant?Recent findings about the complexity of gene function and genome regulation are not taken intoaccount by the concepts of FAO/WHO (2000), Codex Alimentarius (2003) and the EFSA (2006). Their ap-proaches make no difference between unintended effects observed in plants derived from conventionalbreeding and transgenic plants.The presumption of similarity which is the starting point for risk assessment as performed by the EFSAprevents rigorous testing of genetically engineered plants with regard to their safety, predictability andreliability. Even in cases when significant differences between transgenic plants and their counterpartsare observed, they are mostly dismissed by the EFSA as being not of “biological significance”. Spök etal (2004) expose this problem:“In compositional analysis, however, significant differences found are disregarded without attempts toverify or further investigate these differences in order to enhance the likelihood to detect unintendedsecondary effects.”Traavik (2008) raises the decisive question that could finally make the EFSA acknowledge a substantialdifference:“The concept enables the identification of potential differences between a GMO and its unmodifiedcounterpart, and the differences should then be investigated further for potential adverse health andenvironmental effects. But how different can they be before becoming ‘substantially different’?”The hypothesis-driven approach chosen by the EFSA turns around the burden of proof: geneticallyengineered plants are assumed to be safe until the opposite is proven. In addition terms such as “bio-logical significance” are established by the EFSA in a way that leaves a lot of space for speculation andinterpretation and avoids any need for further and more detailed analyses.
Actual standards for risk assessment| Risk Reloaded | 15
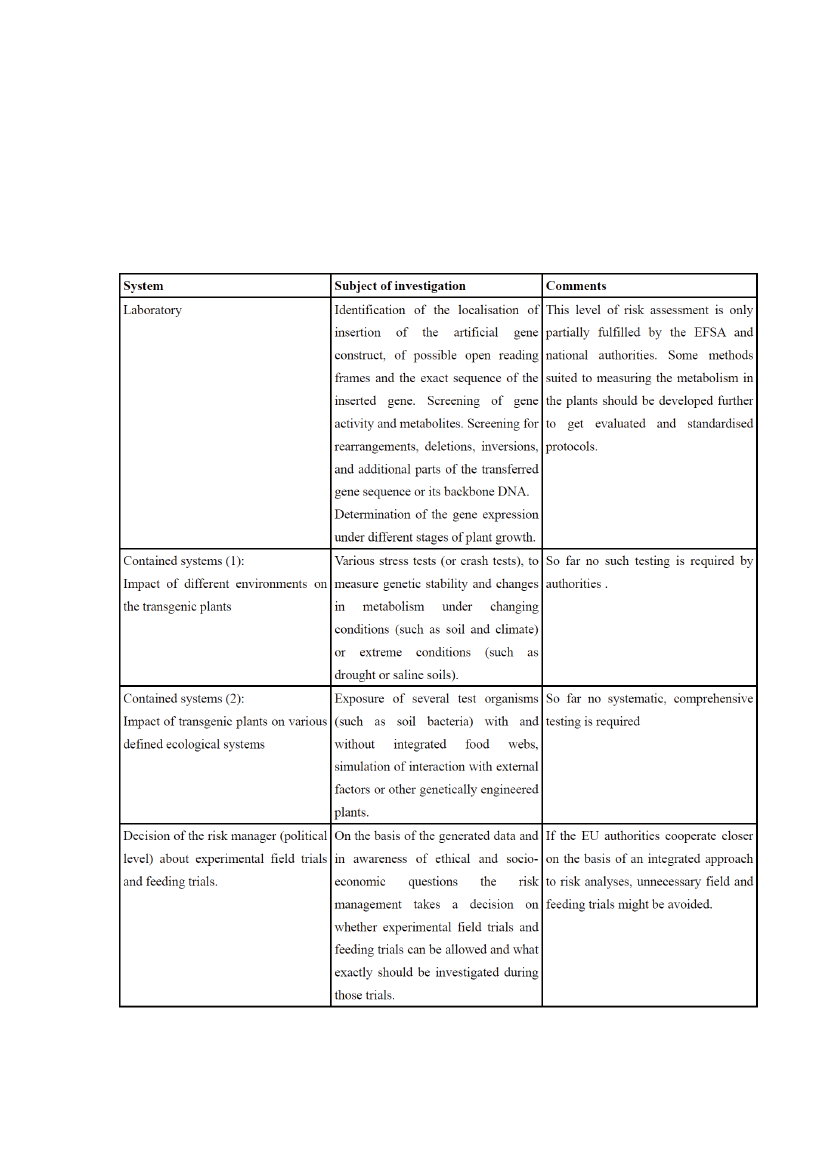
Actual standards for risk assessment andmonitoring in the EUBesides the debate about principles and concepts of risk assessment a decisive question is how theexisting standards are implemented in practise. It was already mentioned that EFSA criteria leave aremarkable space for interpretation and even speculation. Examples of this will be described below. Infact in many cases the personal opinion of EFSA experts seems to be decisive in the question of how andif uncertainties shall be explored further. The guidelines as defined by the EFSA (2006) are anyway inmost of its parts not mandatory and can be handled in different ways from case to case. Regulations forcoherent ‘step by step’ procedures are missing, despite the fact they are required by European legisla-tion (Directive 2001/18, recital 24). While the process for authorisation is indeed organised in severalsteps (first experimental field trials, before application for market authorisation), but the test criteria forthe specific steps are poorly defined and the results not systematically integrated into risk assessment. Asolution could be seen in the introduction of a new regime for risk analyses that gets applied even beforefield or feeding trials take place.
2.
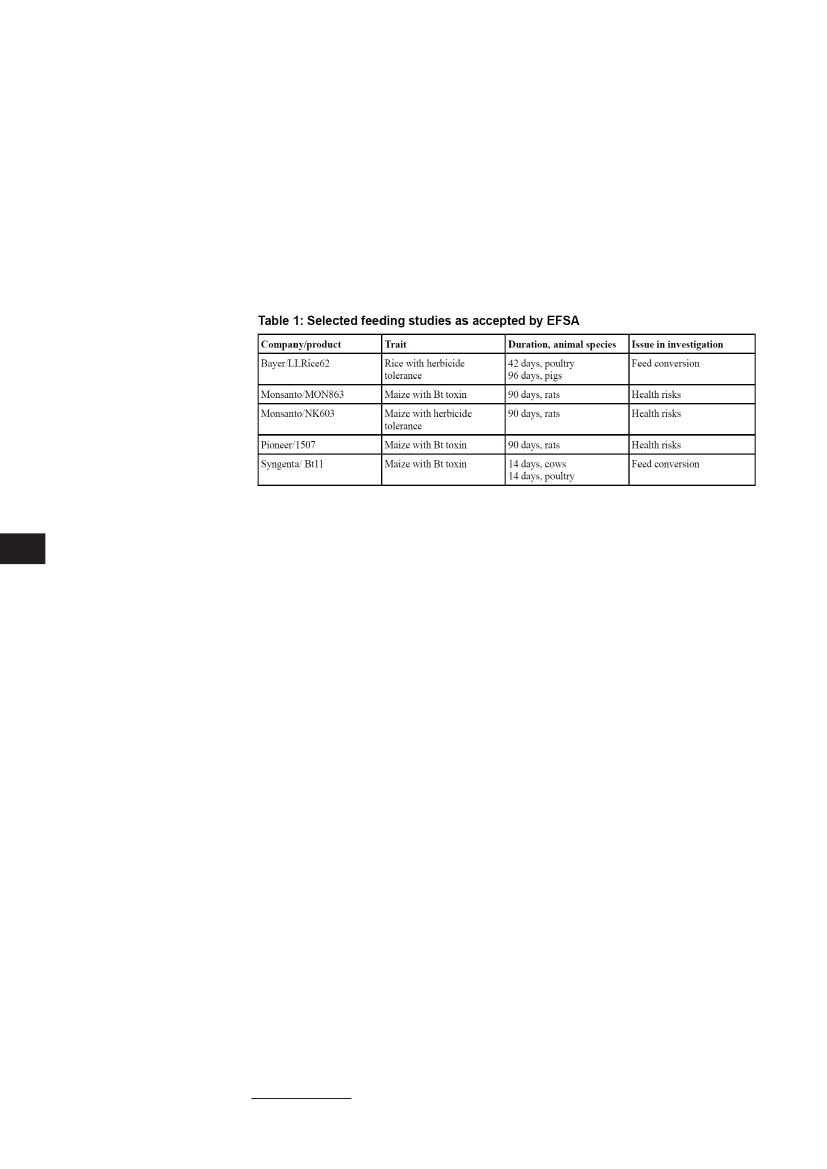
Feeding trialsProblems with the EFSA guidelines get particularly serious in the way the authority deals with feedingtrials meant to investigate potential health effects as already mentioned above. The EFSA does notdemand certain kinds of feeding studies be carried out regularly but decides from case to case (EFSA,2007a, EFSA 2006)By doing so it ignores that several studies concerned with feeding trials have revealed definite signsof negative effects on health (Ewen&Pusztai, 1999; Finamore et al 2008; Kroghsbo et al 2008; Malatestaet al 2002 und 2003; Vecchio et al, 2004; Prescott et al, 2005; Sagstad et al, 2007; Seralini et al, 2007;Valanta&Spök, 2008; Velimirov, et al 2008). While it should be acknowledged that not all aspects ofthe negative effects observed by these authors have been definitively assessed, the existing findingsindicate that feeding trials with the whole plants seem to be quite reasonable and even necessary, beforethese products are authorised for food and feed. Several experts call for mandatory feeding trials. Theseshould include testing with several generations and specific investigations of organs, the immune systemand the reproductive system (Seralini et al, 2009, Spök et al 2004).By analysing the EFSA’s views it becomes evident that the authority in fact accepts dossiers with widelyvarying standards. The feeding trials accepted are substantially different in their methodology andaims. Only in some cases were feeding trials performed that concern potential health risks; in othercases only economically relevant parameters such as feed conversion in farm animals were investigated.Even in the case of the Bayer company’s herbicide resistant Rice LLRice62, which is not meant mainlyfor feed (such as maize and soy) but for human consumption, the EFSA did not request specific feed-ing trials with the whole food in order to investigate potential health risks (see Table 1). More targetedinvestigations that might concern organs, the reproductive system or the immune system were in anycase not requested by the EFSA.Feeding trials with animals such as mammals are a matter of concern from an ethical perspective. Someof the tests proposed imply additional stress for the animals, beyond the uptake of certain feed under labconditions. Since genetically engineered plants and food are rejected or at least seen as being controver-sial by most consumers in Europe, there is a possibility that animal trials will be conducted for productsthat will never become relevant for the EU market.Superfluous feeding trials could, however, possibly be prevented (partially) by the EU Commission. TheCommission, being the risk manager (see also below), may weigh up ethical, economical and scientific
2.1
16| Risk Reloaded |Actual standards for risk assessment
questions within overarching analyses and decisions. To prevent unnecessary animal trials it couldbe the task of the EU Commission to only start a process for market authorisation if a substantial needfor the product can be identified. Such “integrated” concepts, taking into account not only risk relatedissues, are proposed by Haslberger (2006) and Gesche&Haslberger (2006). Within such integratedconcepts some detailed ‘step by step’ procedures can be defined that require certain mandatory checksbefore any feeding trials or releases take place. Some possible features of comprehensive testing arepresented in section 6.
2.2
Ecological risk assessmentDespite the fact that most genetically engineered plants assessed by the EFSA have quite similarcharacteristics, the protocols for investigating their ecological risks differ quite substantially. The dif-ferences concern duration and number of experimental trials, the species investigated that might comeinto contact with the plants, the research protocols and the way the data about exposition are derived.In most cases the data as presented by industry are not suited for drawing final conclusions about thepotential ecological risks of their transgenic plants. Lorch (2005), for example, found a long list of defi-ciencies in the Syngenta company’s dossier of Bt11 maize dossier accepted by the EFSA for cultivation:• during the trials filed, the features investigated were mainly relevant for agronomic and economi-cal purposes but hardly for ecological risk assessment,• most data were not derived under conditions as existing in Europe but were more relevant tothe US, and many of the original data were never published and are not available to interestedmembers of the public,• the concentration of the Bt toxin varied across broad ranges but the reasons for this and the maxi-mal variations were not investigated, and• despite the fact that the plants not only produced an insecticide, but were also tolerant of a herbi-cide, no investigations were required about possible interactions.According to official statements the EFSA so far does not have a coherent concept for ecological riskassessment. New guidelines will be proposed by the EFSA in March 2010 as requested by the EU Com-misison.10The EFSA nevertheless keeps on presenting opinions about the cultivation of genetically engi-neered plants and their associated ecological risks even before these guidelines have been presented.When investigating environmental risks there is a basic dilemma which is similar to ethical problemswith animal feeding trials. Releases made to investigate potential ecological risks can in themselves al-ready present ecological risks. In theory it is necessary to perform experimental field trials for severalyears in all the regions of the European Union which show specific ecological or climatic properties orother relevant environmental conditions, before the ecological consequences of commercial cultivationcan be assessed.
10
http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/envir/104553.pdf
Actual standards for risk assessment| Risk Reloaded | 17
Thus strategies should be developed to avoid experimental field trials that would be made too early,too riskily and even unnecessarily. For this purpose comprehensive investigations in greenhouses,the laboratory or other systems that allow contained experimentation are the best options for obtain-ing more data about product quality and safety aspects. It is true that certain complex investigationscan only be conducted in the open. But it is also true that certain information can in many cases onlybe obtained when work is done under conditions as apply in the lab, as these allow more precise testrequirements to be met.There are a whole number of tests that can usefully be conducted before any release trials. They in-clude simulation of specific environmental conditions and exposition to certain stress factors that mightalter the metabolism in cells and the concentration of key components in the plants. The potentials ofthese test systems are far from being explored by current risk assessment. Several of them will need tobe elaborated further before they can be validated.Most companies show an interest in releasing their genetically engineered plants into the environmentas soon as possible. But under environmental conditions too many factors occur in parallel that cannotbe investigated in detail. Thus it is current practice only to take into account very few indicators dur-ing field trials, with these then used to draw very general conclusions about the safety of the plants (aconcept called the tiered approach by the EFSA). This might help companies to save money and time,but might become quite costly from the perspective of the precautionary principle.Systematic testing which follows a true ‘step by step’ procedure, aiming first to conduct comprehensiveinvestigations and assess a fixed set of data before any releases (or feeding trials) can take place, is notin place in the EU, despite being generally requested by Directive 2001/18. The authorities of the EUmember states are not defining what has to be checked before (or during) experimental field trials. Thedata as made available by the companies are not subjected to an EU wide evaluation protocol.Thus it is proposed here a new, transparent and clearly defined ‘step by step’ procedure be established,before market authorisation is requested and field trials take place or feeding trials are conducted. Thisprocedure should involve the levels of both EU member states and the EU authorities and should alsoinclude assessment of ethical and socio-economical criteria (see section 6).
MonitoringThe deficiencies in risk assessment mentioned also impact the way monitoring is performed. EU regula-tions require post market monitoring of genetically engineered plants. According to Council decision2002/81, genetically engineered plants (and derived products) also have to be monitored for potentialhealth effects and ecological risks. On the aims of risk assessment:“The environmental risk assessment aims, on a case by case basis, to identify and evaluate potentialadverse effects of the GMO, either direct and indirect, immediate or delayed, on human health and theenvironment arising from its placing on the market. This assessment may also need to take account ofpotential long-term effects associated with the interaction with other organisms and the environment.The evaluation of such potential adverse effects should be founded on common methodology based onindependently verifiable scientific evidence.”[...]“Against this background, it is foreseen that the objectives of post-market monitoring, as detailedunder Annex VII, are to:- confirm that any assumptions regarding the occurrence and impact of potential adverse effects of theGMO or its use in the environmental risk assessment are correct, and- identify the occurrence of adverse effects of the GMO or its use on human health or the environmentwhich were not anticipated in the environmental risk assessment.”
2.3
18| Risk Reloaded |Actual standards for risk assessment
The way monitoring is actually performed depends essentially on the results of risk assessment. If noconcrete risk is identified, monitoring might only be performed as ‘general surveillance’ without anyspecific scientific investigations. So the risk assessment and the system for monitoring move in circles.The risks of genetically engineered plants are not thoroughly investigated during risk assessmentand therefore not subjected to detailed monitoring. One could say in summary that the aims of the EUregulations (to prioritise the precautionary principle) produce the opposite as a result of this system ofself-reinforcing failures.In fact the EFSA has not so far proposed case specific monitoring for any genetically engineered plant.The only exception to this is that during cultivation of Bt plants a case specific monitoring shall beestablished in order to observe the potential emergence of resistant pest insects. But this kind of moni-toring is much more motivated by the economical interests of the seed companies (which might have toface falling turnover rates if their crops start failing) than by the need for a survey of ecological risks.The EU Commission is well aware of current deficiencies in the monitoring of genetically engineeredplants – at least with regard to potential health effects. As the Commission stated in 2005 (EuropeanCommunities, 2005):“As regards food safety, even if some GM products have been found to be safe and approved on alarge scale..., the lack of general surveillance and consequently of any exposure data and assess-ment, means that there is no data whatsoever available on the consumption of these products – whohas eaten what and when. Consequently, one can accept with a high degree of confidence that thereis no acute toxicological risk posed by the relevant products, as this would probably not have goneundetected – even if one cannot rule out completely acute anaphylactic exceptional episodes. However,in the absence of exposure data in respect of chronic conditions that are common, such as allergy andcancer, there simply is no way of ascertaining whether the introduction of GM products has had anyother effect on human health.”11The EFSA, too, admits (2007a) that no adequate systems for deriving relevant epidemiological data arecurrently established. But according to EU regulations, market authorisation of genetically engineeredplants (and derived food and feed) is closely connected with the availability of effective monitoringsystems.The EFSA is not in fact responsible for deciding on how to monitor and survey potential risks (it canonly make some proposals in the matter). Responsibility falls within the sector of risk managementand political decision making, which in this case is part of the remit of the European Commission. As aconclusion one could question if the Commission is acting in accordance with European regulations ifmarket access for genetically engineered food and feed is granted despite it being known that effectivesystems for monitoring its potential health effects are not available.
11
Paragraph 45, European Communities (2005)
New doubts about safety| Risk Reloaded | 19
New doubts about safetyGiven the deficiencies of existing risk assessment it is not surprising that even the safety of productsthat have already passed authorisation is still a matter of controversial discussions. This is mostevident in the case of genetically engineered maize MON810, the cultivation of which is prohibited by agrowing number of member states in the EU.
3.
3.1
Plants producing insecticidesBy 2009 the cultivation of insect resistant maize MON810 had been prohibited in a growing number ofEU member states: Austria, France, Germany, Greece, Hungary and Luxembourg. So far this Bt toxin-producing maize is the only genetically engineered crop allowed to be commercially cultivated withinthe EU. It is grown on about 100,000 hectares in Spain.A modified gene sequence from the Bacillus thuringensis soil organism was inserted into the plants bygenetic engineering. This causes the Bt toxin to be produced in all parts of the plants. From there it isalso emitted via pollen and parts of the plants into the environment and secreted by the roots into thesoil. MON810 was granted market authorisation for the EU in 1998; this ended (according to EU regula-tions) in 2007. The US company filed an application for reauthorisation which was assessed and decidedon in its favour by the EFSA in June 2009 (EFSA, 2009).The German government prohibited the cultivation of MON810 in April 2009. It was argued that newpublications showed negative effects on organisms such as ladybird larvae (Schmidt et al., 2008) andwater fleas (Bøhn et al. 2008). An overview of recent scientific publications shows that the effectsobserved on these and other non-target organisms indicate a general problem with MON810 - there isgrowing evidence that the supposed selectivity of the toxin (which is meant to be active only on certainLepidoptera larvae) is not in accordance with the scientific facts (Then&Brockmann, 2009).Because the natural occurring Bt toxins show a quite complex mode of action it was supposed that thetoxins as produced in the plants can only fulfil their deadly mission under conditions as met in the gutof the larvae of certain insects (Lepidoptera). Specific receptors, described as ‘target organisms’, occur-ring in the gut of these pest insects (and in the case of MON810 the corn borer especially), are neededto activate the toxin. In contrast so called ‘non-target organisms’ are supposed not to be endangeredbecause those receptors cannot be found. But this general assumption has to be reassessed in the lightof recent publications:• The toxin as produced in the plants is different in its structure from the natural occurring Bt toxinand thereby also changed in its biological activity (Hilbeck&Schmidt, 2006; Li et al., 2007).• It seems premature to declare that the mode of action as described in target organism is the onlyway to initiate the toxic effects. Several publications show that a coherent theory for the modeof action of the Bt toxin (and the role of receptors) is missing. The different existing models arepartially complementary but also contradictory to each other (Pigott&Ellar, 2007).• Inference and synergies with external factors which can enhance the toxicity of the toxins haveso far not been assessed. Recent research shows that the selectivity and the efficacy of the Bttoxins can be quite substantially influenced by external factors (Kaatz, 2005, Kramarz et al. 2007,Broderick et al., 2009, Then, 2009b). These potential risky synergies are also discussed in the inter-view with a honey bee expert in the next section.These aspects are very relevant in the risk assessment of Bt plants such as MON810, but are ignoredby the EFSA in their recent assessment of the application for reauthorisation of MON810 (EFSA, 2009).
20| Risk Reloaded |New doubts about safety
The EFSA does not question the selectivity and mode of action of the Bt toxin as produced in the plantswhen listing various publications about effects on non-target organisms. Figure 1 gives an overviewof some of the questions concerning the mechanisms of Bt toxins (source: Then, 2009b). It shows thatmany decisive questions concerning the toxicity of Bt toxins still need to be answered.(a): The inactive form of the protoxin isproduced by Bacillus thuringiensis. In thegut of the insect larvae it is transformed toa solubilised active toxin which is shorterthan the protoxin. For this process of activa-tion an alkaline pH and certain enzymesare needed. These steps are not necessaryfor Cry toxins as produced in geneti-cally engineered plants which are alreadyactivated by the technical process and byplant enzymes. (b): Several types of midgutreceptors are thought to be decisive forthe binding of the toxins in cells and someexperts question the role of receptors ingeneral. (c) Several mechanisms are thoughtto be decisive in the last step of toxin reac-tion:.Models exists with and without poresin the pithelial cells and with and withoutinvolving gut bacteria (source: Then, 2009b)
Another detail in the EFSA’s recent opinion on MON810 (EFSA, 2009) is quite typical of the way theauthority deals with uncertainties. Rosati et al. (2008) report that at the border between the normalmaize genome and the newly inserted gene sequence a hybridisation of the DNA takes place leadingto unexpected biological activity. These hybrid genes could even cause the production of proteins thathave no similarity with known plant compounds. The EFSA takes notice of the publication but does notsee any need for further investigations:“These putative recombinant proteins did not show homology with any known protein and do not raiseany new safety concerns.”It looks as if just because the properties of the new putative proteins are not known, the EFSA consid-ers them as being safe (see also Cotter&Mueller, 2009). This is a typical example showing how biasedthe EFSA is in dealing with uncertainties that show up during risk assessment of genetically engi-neered plants. Uncertainties are mostly judged as indicating no or only minor risks because of theunderlying hypothesis that defines transgenic plants as being comparable (or one could say similar) toconventional plants which have a history of safe use.
3.2
Herbicide-tolerant plantsTransgenic plants tolerating the glyphosate (brand name: Roundup) herbicide are grown in severalregions of the world. The impact of the herbicide is increasingly cause for debate. The amount of thisherbicide used has been rising for years, since more and more weeds show some tolerance againstglyphosate and higher dosages are needed to treat them effectively (Service, 2007). As more is used,residues of the herbicide in the plants are also likely to increase, and some countries have alreadychanged the level of acceptable uptake in food. At the same time more and more publications showthat the mixture of the herbicide as it used in Roundup can cause negative effects on human embryos(Benachour&Séralini 2008) and the reproductive system (Gasnier et al, 2009).This has been a matter for political discussions in Argentina, in particular, where about 100% of soy-beans cultivated are herbicide-tolerant to glyphosate. After reports that glyphosate is more highly toxicfor amphibians than previously thought were published thereglyphosate, health risks were also newlydiscussed. A group of attorneys active on environmental questions started a legal procedure to with-draw the authorisation of the herbicide.12Other environmental impacts of the large scale cultivation12Webber, J., Weitzman, H., 2009, Argentina pressed to ban crop chemical after health concerns, Financial
New doubts about safety| Risk Reloaded | 21
of the herbicide-tolerant soybeans have also been reported – impacts on soil organisms and the loweravailability of essential soil minerals and aquatic systems (see overview Mertens, 2008).The increase in herbicide-tolerant weeds has given reason for several companies to go for the produc-tion of further herbicide tolerant plants that can resist other chemicals (Service, 2007). Indeed newcompetition in the fields that is likely to result in the usage of chemicals with a higher toxicity beingapplied on large scale has started. This can be seen for example by the plans of US company, Dow Agro-Sciences, for arming soy plants with resistance against the 2,4 D herbicide (2,4-Dichlorphenoxy).13Thischemical is supposed to have much higher detrimental impact on environment and human health thanthe current use of glyphosate. 2,4 D was also a compound in Agent Orange, used in the Vietnam war todefoliate trees and severely damaging human health. All in all the use of herbicide tolerant plants doesnot lead to a reduction of the use of herbicides; on the contrary, thetoxic burden on human health andthe environment will be increased.
‘Stacked events’ and interactions betweentransgenic plantsGenetically engineered plants that are manipulated to combine different gene constructs are called‘stacked events’. In 2009 a maize was authorised with eight different gene constructs in the US andCanada.14The plants are a joint production of Monsanto and Dow; they are sold under the brand nameSmartStax and are supposed to be grown on several million acres within the next few years. The plantsproduce several insecticides and are tolerant of two herbicides.The EU has also authorised several stacked events for import (such as NK603xMON810 and MON863x-MON810). With positive opinion on Bt11 and 1507 maize the EFSA favours the cultivation of transgenicmaize in the EU which combines insect resistance and herbicide tolerance (EFSA 2005a and b).The EFSA guidelines for the risk assessment of stacked events do not foresee specific investigationsto identify unintended interactions between the different gene constructs and their products (EFSA2007b). The EFSA assumes that in most cases the assessment of each of the single constructs will besufficient. The authority is aware of potential interactions between the different events (EFSA 2007b)but concludes that field trials that have already been performed for one year will be enough to identifyunintended effects. In doing so it is in the main following the industry’s guidelines. Monsanto for ex-ample refers to the notion that experience with conventional cultivation should be drawn on in dealingwith plants and stacked events.“An important point for our work on stacked events is that conventional breeding is not necessar-ily safe, but it has a history of safety developed over time. In addition, we can use our knowledge ofbreeding to produce stacked events that meet criteria of acceptable safety (as safe as).”15The Austrian authorities are among others who have assessed this approach by the industry, which isin the main also applied by the EFSA, as inadequate (Spök et al., 2007). The EFSA is in principle boundby law to examine, and also take account in its monitoring, interactions between plants and cumulativeeffects when dealing with genetically engineered plants. This can be seen from Directive 2001/18:
3.3
Times, May 29 2009, http://www.ft.com/cms/s/0/3d74344c-4be8-11de-b827-00144feabdc0.html?nclick_check=1, Argentinien: Kranke Dörfer, Gesundheitskrise durch herbizidintensive Sojaproduktion,5.03.2009, BUENOS AIRES, IPS EUROPA, http://www.ipseuropa.org/index.php,ARGENTINA: Soy - High Profits Now, Hell to Pay Later (29.07.2009, Buenos Aires, IPS International)http://www.ipsnews.net/news.asp?idnews=43353131415http://www.gmfreeze.org/page.asp?id=385&iTypehttp://www.bloomberg.com/apps/news?pid=20601103&sid=a57J5HHLMOg4Thomas Nickson, Monsanto Company, USA, 2009-07-10, http://bch.cbd.int/onlineconferences/stacked2_ra.shtml?threadid=1214
22| Risk Reloaded |New doubts about safety
“A case-by-case environmental risk assessment should always be carried out prior to a release. Itshould also take due account of potential cumulative long-term effects associated with the interactionwith other GMOs and the environment.” (recital 19)and“Monitoring of potential cumulative long-term effects should be considered as a compulsory part ofthe monitoring plan.” (recital 20)Annex II of the 2001 Directive also explicitly mentions interactions between genetic engineered plantsand cumulative effects. Cumulative effects and potential interactions have to be taken into account aswell in the parallel cultivation and imports of different genetically engineered plants and in the case ofstacked events in single transgenic plants. As already mentioned it is known that synergies can emergebetween different Bt toxins (Schnepf et al, 1998). Investigations by Kramarz (2007) and Kaatz (2005)show that synergies and interactions with external factors can make Bt toxins toxic in non-target or-ganisms. Further, there are concerns that combined cultivation of Bt plants can cause cross resistancein pest insects (Tabashnik et al, 1997 and 2009). This means the EFSA would have to consider possibleinteractions between MON810, Bt11 and maize 1507 and their Bt toxins if these transgenic plants aregrown or fed to animals in any combination. The same is true for stacked events such as MON863x-MON810.Interference between Bt producing plants and the use of chemicals (herbicides, pesticides) is demon-strated as well. It has been published that the additional use of insecticides impacts the concentrationof Bt toxins in the plants (Griffiths et al, 2006). Further, if Bt toxins are used in combination withherbicides such as glyphosate and glufosinate, the herbicidal residues in the soil will decrease slower(Accinelli et al. 2004). The potential interactions between events conferring herbicide tolerance andinsect resistance are relevant for several genetically engineered plants such as Bt11, maize 1507,NK603xMon810 and SmartStax.According to a report by the EU Joint Research Centre (JRC, 2009) it can be expected that more than100 different events might be introduced into markets (or have authorisation applied for) in the nextfew years until 2015, and that several hundreds or even thousands of possibilities will be created forcombining these events in stacked plants. According to EFSA standards detailed analyses of potentialinteractions or cumulative effects will only occur in some rare cases. The arguments presented aboveimply these standards should be seen as conflicting with current EU regulations such as Directive2001/18.
3.4
General risks from genetically engineeredplantsRisks from genetically plants depend not only on certain traits or events, but are inherently caused bythe method of their production. While it is tru that risk assessment has to be performed on case by casestudies, it also cannot be denied that there are certain risks that are typical just for transgenic plants.
3.4.1
Risks to immune systemPrescott et al (2005)’s publication shows that the technology of invasive gene transfer can cause severerisks to human health. A gene sequence from beans was transferred into peas. Investigations with thetransgenic peas resulted in a severe reaction of the immune system in mice. Interestingly the trans-genic peas were tested before on rats, pigs and poultry without showing these detrimental effects. Onlytesting specifically in mice revealed the immunotoxilogical reaction. Valenta &Spök (2008) evaluate thefindings once more and identify some methodological deficiencies by the experiments as performed byPrescott et al (2005). They discuss the possibility that additional proteins that might have contributed
New doubts about safety| Risk Reloaded | 23
to the immune reaction were produced in the peas. They conclude that the causes of the observedeffect are not known in detail (therefore it is not clear to what extent the protein from the bean bears aspecific immanent risk) and explain that in the light of these findings the guidelines for risk assess-ment in the EU should be revised:“On the other hand, the Prescott study clearly shows that a transgenic protein can induce undercertain conditions an unwanted immune response leading to organ pathology and certain experimentsdemonstrate that exposure to the transgenic protein can increase the immunogenicity of other unre-lated proteins which are administered together with the transgenic protein, a finding which wouldnot be detected by current risk assessment procedures. In this context the Prescott study indicates astrong need for reconsidering the current approach to GM allergenicity assessment.”So far there are no standardised methods for testing for immune reactions to transgenic plants (EFSA,2007a). This should certainly be a matter of concern, because there are several other publicationsbesides Prescott (2005) and Valenta&Spök (2008) which show specific risks just at this point (Finamoreet al., 2008; Kroghsbo et al.,2008; Sagstad et al., 2007).
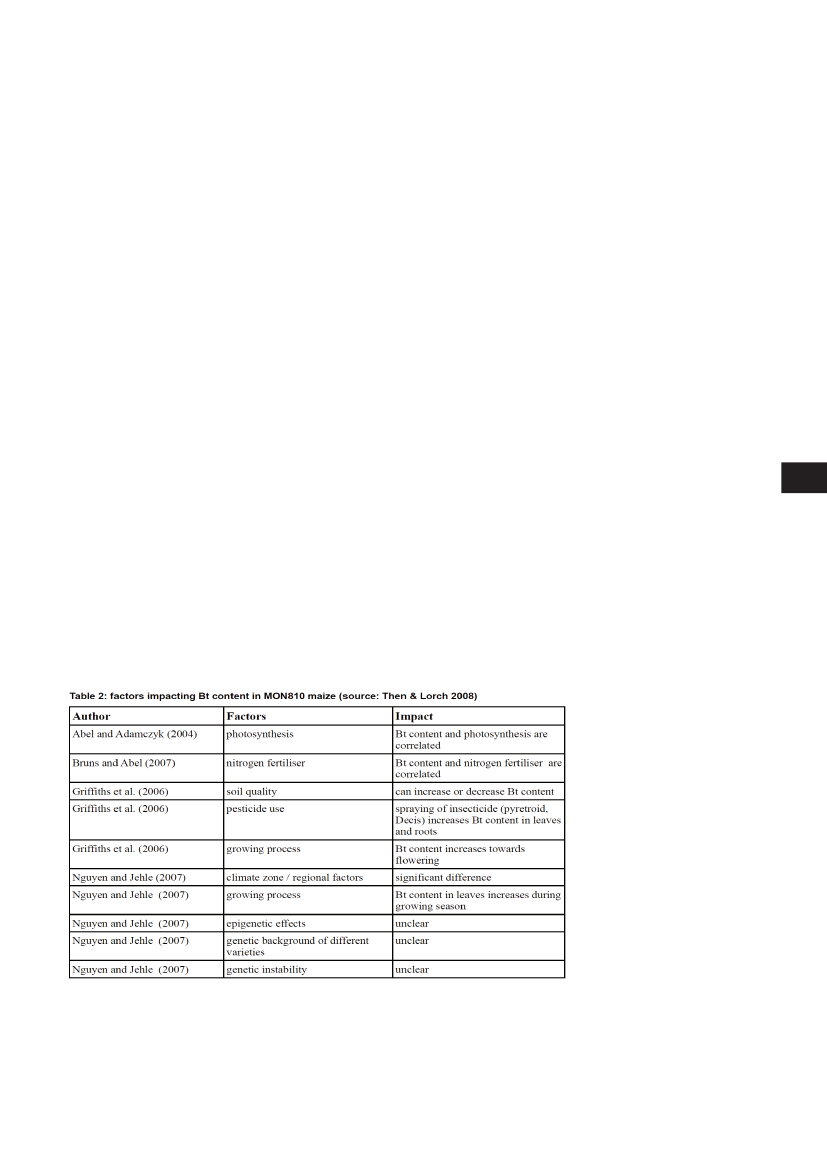
How the environment impacts transgenicplants and the introduction of crash testsFindings in genetically engineered maize and cotton producing the Bt toxin show that its associatedrisks are dynamic and subject to environmental conditions. Interference from light, fertilizer and cli-mate can affect the Bt content in maize MON810 substantially (see table 2). Further factors in the plantsseem to play a role because significant differences between maize plants grown side aside in one fieldhave also been observed (Lorch&Then, 2007). All in all there are not yet enough results published tojudge the real range of possible variations of Bt content in transgenic plants. In addition the methodol-ogy and protocols used for measuring Bt content are not standardised and thus the results can hardlybe compared in many cases (see Then&Lorch, 2008).
3.4.2
It is also known from experience with genetically engineered cotton (Chen et al., 2005) and soy(Coghlan, 1999, Gertz et al., 1999) that transgenic plants react to climate conditions. Furthermore,investigations on genetically engineered potatoes show that under stress conditions these plants reveal
24 | Risk Reloaded |New doubts about safety
unexpected reactions which are not detected under ‘normal’ conditions. Matthews et al (2005) subjectedgenetically engineered plants to several biotic (such as infections) and abiotic stress factors. They foundseveral differences between the potatoes in the formation of of defending compounds. Their conclusionreads:“Transgenic and nontransgenic potato lines were exposed to a range of biotic and abiotic stresses anda range of environmental conditions in the field and during storage. After the stress had taken effect,a comparison was made between the two groups of the potato glycoalkaloid and sesquiterpene levels.Significant differences were observed in the levels of both in the transgenic and control material andin infected and non-infected material.”The EFSA does not request any data on the potential (extreme) impact of environmental conditions ontransgenic plants. While it is interested in some information about the genetic stability of the artifi-cially introduced gene construct over a period of several generations, the EFSA does not define anyexternal conditions which should be met for testing stability. The question of how the metabolism of theplants might react to climate stressing, for example – an issue which seems to be quite relevant in thecontext of ongoing climate change – is not part of the risk assessment. Because genetically engineeredplants have to be seen as technically derived products, it is quite plausible that specific crash testsmight reveal some limits of their genetic and metabolic stability. In the report as presented here it issuggested specific tests – ‘crash tests’, as they are called - be introduced for measuring the impact ofdefined environmental conditions on genetically engineered plants (see section 6).Introducing such tests would make it possible to generate a great deal more basic data on each productthan at present before they are released or applications for approval are made. The stability of trans-genic plants and possible changes in their metabolisms can only be determined with defined stressconditions in a contained system. This kind of controlled test (crash test) would not only provide dataon the concentration of critical compounds such as Bt insecticide, it would also allow observation of theplant’s own compounds whose concentration may inadvertently have been influenced by the introduc-tion of an additional gene.There are now a number of new test procedures developed in recent years which can be used. For ex-ample, so-called microarrays allow simultaneous measurement and compilation of metabolic profiles ofseveral gene activities. These tests are carried out internally by various companies but the results arehardly ever published. The EFSA believes these investigations to be important but does not considerthem to be ready to be put into practice, although such procedures have been available for years now(EFSA 2007a).Besides these newer methods of investigation there are many other ways of determining changes in thecompounds of genetically engineered plants such as the content of Bt toxins. This does, however, neces-sitate an agreement between laboratories on the relevant standards.Only when the data is presented can useful hypotheses be formulated to describe how plants behaveunder changing or extreme environmental conditions, the consequences for plants and the risksinvolved.
How independent are public research and the authorities?| Risk Reloaded | 25
How independent are public research andthe authorities?In the EU the promotion of independent research on genetically engineered organisms is safeguardedby Directive 2001/18:“Member States and the Commission should ensure that systematic and independent research on thepotential risks involved in the deliberate release or the placing on the market of GMOs is conducted.The necessary resources should be secured for such research by Member States and the Communityin accordance with their budgetary procedures.”Despite this there have been many complaints in the last few years that independent research inEurope is systematically hampered by several mechanisms.16This problem was recently brought up inan interview conducted by Christof Potthof with a German honey bee expert, Hans-Hinrich Kaatz. Thescientist had disturbingly realised that staff of the US company Monsanto knew about a decision byNature magazine to reject his publications before he the author was contacted. Parts of the intervieware documented below.17In 2009 statements that public research is systematically hampered by seed companies were publishedin the US. 26 scientists from 16 US states in which genetically engineered maize is commercially grownfiled a complaint at the EPA. They reported that the industry was denying access to necessary researchmaterial by abusing intellectual property rights.18Under these conditions truly independent researchcannot be conducted and important questions concerning the impact of the cultivation of transgenicplants cannot be investigated19:“Technology/stewardship agreements required for the purchase of genetically modified seed explicitlyprohibit research. These agreements inhibit public scientists from pursuing their mandated role onbehalf of the public good unless the research is approved by industry. As a result of restricted access,no truly independent research can be legally conducted on many critical questions regarding thetechnology, its performance, its management implications, IRM, and its interactions with insect biol-ogy. Consequently, data flowing to an EPA Scientific Advisory Panel from the public sector is undulylimited.”The editors of Scientific American Magazine report:“But agritech companies such as Monsanto, Pioneer and Syngenta go further. For a decade their useragreements have explicitly forbidden the use of the seeds for any independent research. Under thethreat of litigation, scientists cannot test a seed to explore the different conditions under which itthrives or fails. They cannot compare seeds from one company against those from another company.And perhaps most important, they cannot examine whether the genetically modified crops lead tounintended environmental side effects.”20These statements are especially relevant because in the debate about potential risks of transgenicplants the US is very often referred to as an example for how no adverse effects from large scale grow-ing are so far known.Scientists in Europe, too, complain of restrictions to access, or of even no access, to research material.Two different kinds of material are relevant here: Genetically engineered plants are covered by patent
4.
1617181920
Kleiner Käfer, große Fragen, Frankfurter Allgemeine Zeitung, 19. April 2009Full version only in German: http://www.gen-ethisches-netzwerk.de/gid/194/kleiner-parasit-grosse-wirkungNew York Times 20.Feb. 2009: Crop Scientists Say Biotech Seed Companies Thwarting Research on GMO Safety, Efficacy, http://www.nytimes.com/2009/02/20/business/20crop.htmlhttp://www.regulations.gov/fdmspublic/component/main?main=DocumentDetail&o=090000648084de39Do Seed Companies Control GM Crop Research? Scientific American Magazine - August 13, 2009 http://www.scientificamerican.com/article.cfm?id=do-seed-companies-control-gm-crop-research&print=true
26 | Risk Reloaded |How independent are public research and the authorities?
law, which is the basis for written agreements required for their purchase. Secondly, there is the mate-rial needed to make comparisons between the transgenic plant and its conventional counterpart, theso called isogenic lines, access to which is even more difficult. These plants are the closest ‘relatives’ tothe genetically engineered plants and a prerequisite for assessments that aim to investigate substantialequivalence.Independent researchers also report difficulties in publishing their findings if these are in contradictionto the interests of the industry. An example of these complications is the history of a publication aboutresearch on transgenic seed contamination of maize landraces in Mexico, one of the centres of origin ofmaize biodiversity. As long ago as 2001 initial findings were published about contamination in regionswhere seed propagation of local varieties takes place (Qist&Chapela, 2001). These findings were chal-lenged by many sides and even scientists involved were afraid of becoming removed from their publicinstitutions in the US. Some contamination was again detected in 2008. But the Proceedings of theNational Academy of Sciences magazine rejected publishing these results, because it was afraid thatfurther political debates might arise (Dalton, 2008). As a scientist, Alison Snow, from the US ColumbusUniversity wrote in an article for Molecular Biology magazine21:“Furthermore, the politically sensitive nature of this information has made it difficult for researchersto publish their findings.”This case is not the only one. There are several publications that caused real hunts against their au-thors because their findings revealed potential risks from genetically engineered plants. For example,in September 2009, Nature magazine22reported about the case of Rosi-Marshall, which became thetarget of a campaign just because she described the impact of Bt plants on some water organisms (Rosi-Marshall et al., 2007):“Behind the attacks are scientists who are determined to prevent papers they deem to have scientificflaws from influencing policy-makers. When a paper comes out in which they see problems, they reactquickly, criticize the work in public forums, write rebuttal letters, and send them to policy-makers,funding agencies and journal editors.”
4.1
An illustration: feeding trials with cows andthe dairy industryA research project involving feeding trials with the transgenic maize MON810 illustrates how difficultthe conditions for independent research into risks in Germany are. Its results were presented by theTechnical University of Munich (TUM) in 2009 (Meyer et al., 2009), in the midst of political discussionsin Germany about the prohibition of the cultivation of MON810 maize. According to scientists involvedin this feeding trial, which lasted 25 months, the findings can be taken as proof that the transgenicmaize has no impact on lactating cows. It further highlighted that no residues from the transgenicmaize could be detected in the animals’ meat or milk. The study was presented to the press as the beststudy ever made:“The study by Munich Technical University, the most detailed and most precise study ever conductedworldwide, has shown yet again that feeding with transgenic maize does not have any impact on thefood chain.”23The results were also published in an international magazine (Steinke et al, 2009). The publication men-tions that the findings are of robust quality because of the long duration of the feeding trials:“Feeding Bt maize over a period of 25 months had no effects on the performance and metabolic param-eters in this study. The statistical differences between isogenic and transgenic fed dairy cows (milk212223Molecular Ecology, 2009, 18, 569–571Waltz, E., 2009, Battlefield, Nature Vol 461/3 September 2009: 27-32Prof. Dr. Wolfgang Herrmann, president of the Technical University Munich, quoted in Landshuter Zeitung, page 2,7st Aprile, 2009, tranlation: authors
How independent are public research and the authorities?| Risk Reloaded | 27
protein, milk fat and glucose) in the first lactation were not confirmed in the second lactation and areprobably due to individual or physiological differences between animals.”But as a comparison between the results as presented in German (Meyer et al. 2009) and English(Steinke et al., 2009) shows (Then 2009 c), the international publication does not mention severalrelevant data and furthermore hides the fact that many animals were removed from the trials duringthem. Only one third of the cows (18 animals) were fed over a period of 25 months as stated by Steinckeet al., 2009. The rest of the 54 cows were changed during the trials without concrete reasons or the ex-act timing of the substituting being made public. Despite the fact that changing the animals in this wayis highly plausible as the main reason why significant results could not be confirmed over the wholeperiod of the study, the official international publication does not mention this important fact.Further analyses of the data shows that the study hardly allows any final conclusions to be made. Asmentioned, the statistical figures do not show which animals took part in the feeding trials over whichperiod of time. Specific and detailed investigations concerning certain organs, as well the examinationof the calves, are missing. Further, it is not clear to which extent the Bt toxin was deactivated by theheating process performed for the preparation of the feedstuff. All in all the conclusions of Steincke etal (2009) and the presentation of the results in the press have to seen as being as inadequate and evenmisguiding.The second part of the study, which aimed to detect residues (such as specific DNA) of the geneticallyengineered plants in animal products (especially milk), also has to be discussed in a critical way. Themethods and protocols used are not in accordance with international standards and technically not suf-ficient for showing the highest likelihood of making such a detection successfully (Then, 2009c).The project was funded partially by the dairy industry. Some of its members were repeatedly criticisedby consumer and environmental organisations because they allowed the use of genetically engineeredplants in their animal feed. Even before the trials were started, the leading scientist in the projectsigned a declaration stating that residues from genetically engineered plants cannot be found in animalproducts (see Then, 2009c). All in all this research project provides a lot of good reasons to raise doubtsin the independence of public research.
Special agencies’ networkIn Germany the framing of public research and independence of the national authorities are subjects ofgeneral debate. There are several reasons for these discussions, which were presented in an overviewin 2008 (Lorch&Then, 2008). Some examples of the connections between corporations and the authori-ties documented in that overview are listed here:• • • • A consulting agency which closely works with biotech industry officially presents the results ofpublic risk research (www.biosicherheit.de).There are several reports that staff of the German authorities (Hans-Jörg Buhk and Detlef Bartsch)appeared in a Monsanto promotion video.German authority staff (Joachim Schiemann und Klaus Dieter Jany) were involved in patent ap-plications on genetically engineered organism.German authority staff have been coordinating organisations (such as Wissenschaftlerkreis GrüneGentechnik) that are supportive of the use of genetically engineered plants in agriculture.
4.2
Systematic analysis shows there to be a close interconnection between the authorities and consultingagencies in national and international institutions. Special mention can be made here - besides the Ger-man Wissenschaftlerkreis Grüne Gentechnik of the Public Research & Regulation Initiative lobbying
28 | Risk Reloaded |How independent are public research and the authorities?
organisation as well as the EU’s Biosafenet and Co-Extra projects BiosafenetCo-Extraand the Inter-national Society for Biosafety Research (ISBR). The German consulting agency Genius GmbH can beidentified as one of the most active organisations promoting active networking between the authoritiesand corporations.The report by Lorch&Then (2008) mentions the following activities of Genius GmbH: Genius is mem-ber of the BIO Deutschland national umbrella organisation and the European lobby organisation, theEuropean Federation of Biotechnology. It has about 20 staff and its official annual turnover is about twomillion euro. Genius’s customers are companies like Bayer, BASF, Monsanto and Syngenta, and also theEuropean Commission, the German state of Hesse and the German ministry for education and research(BMBF). Public outreach often is presented indirectly: the consultancy edits the biosicherheit.de websiteon behalf of the BMBF and presents the outcome of publicly funded risk research – at a first glance thewebsite looks like an official medium of the ministry.A second project that aims at public outreach and is organised with the support of Genius is the “GMOCompass”, which was first financed as a EU project (2005-07), then funded by EuropaBio (2007) and theGerman agriculture ministry (2007-2008). Other organisations involved in the project are the initiatorsof the transgen.de website, which was first financed by a consumer organisation and then partiallyfinanced by the biotech industry. Intended to be perceived as having a close relationship to a consumerorganisation, GMO Compass gives the impression of being an unbiased institution. But the editing teamis more or less identical to that of www.biosicherheit.de, describing themselves as “independent sciencejournalists”. Joachim Schiemann of the Julius Kühn institute (JKI) and former member of EFSA’s GMOpanel is also mentioned as are a member of GMO Compass’ advisory team and the industrial umbrellaorganisation EuropaBio.Projects such as biosicherheit.de and the GMO Compass can be seen as an exception in the activitiesof Genius GmbH. Mostly its members work as service-orientated consultants, very often in combina-tion with members of the authorities. The JKI’s Joachim Schiemann, in particular, is very often to befound in this connection.24Genius was active in the EU’s Biosafenet project, its coordinator JoachimSchiemann. Genius staff are involved in the ISBR’s internet presentation, while its former president wasSchiemann.25Joachim Schiemann was a partner in the EU’s Co-Extra project, with Genius working asan editorial office. Together with Schiemann and Jeremy Sweet (EFSA) Genius was also a member of themanagement board of Co-Extra. Joachim Schiemann was the German representative at the EU “Plantsfor the Future” strategy meeting and members of Genius were invited as experts. Genius was also ac-tive for the EFSA editing its publications such as the 2006 annual report.Agencies such as Genius GmbH work like an intermediary between the authorities and industry; theylower the borders between public institutions and private interests and foster increasing intranspar-ency in the way decisions are really taken where genetically engineered plants are concerned.
2425
most details as found in the year 2008Schiemann was followed by Patrick Rüdelsheim, former staff member of Plant Genetics Systems and Bayer CropScience.
Interview| Risk Reloaded | 29
Interview by Christof Potthof with the beeresearcher Hans-Hinrich Kaatz, University ofHalle26IntroductionIn an investigation colonies of honey bees infected with the parasite nosema and fed with the pollenof the genetically modified maize MON810, collapsed much earlier than those colonies which were fedwith conventional maize pollen. (......)What did you observe during the investigation?In the first year of the field trial designed to last six weeks in that year, the honey bee colonies fed withthe Bt maize pollen very clearly collapsed after three weeks. The effects were always the same in theBt nets. I found this very unsettling because it was not what I had expected; all the previous data fromother researchers, which is naturally not transferable, had suggested that the Bt toxin had no effect onhoney bees. Then, of course, one has to think about why this should be. It was possible that the causescould be found in our methods; we had used a ten-fold higher concentration of the Bt toxin than therewould have been in a natural environment i.e. the Bt content given as the content in the pollen of thegenetically modified plants. Because we had not expected any effect we thought we would “use the ten-fold amount to be on the safe side. Also if we used the ten-fold amount and found nothing then we couldput our minds at rest about the lower content in the plants.” (.....)Did you find any other clues when you examined the dead honey bees?Well, of course, we asked ourselves what had happened to the bees. There were dead honey beeseverywhere. We tried to find out which factors had caused their deaths. One possible factor was thataffliction with nosema27was relatively high in the honey bee colonies. That was something we had notexpected quite so strongly at that time in autumn. Basically we knew that the occurrence of nosemacan be intensified under stress conditions.If the higher occurrence of nosema is connected to stress then it should have appeared inthe colonies without Bt.Yes, that is how it was. We examined the control colonies without Bt and found no differences. But itwas clearly observed that first of all only the Bt group of colonies collapsed and the control group col-lapsed later on. However, I need to repeat that we have no evidence. At the moment it is nothing morethan a correlation, it could be coincidence.But does it have statistical significance?Yes. That is indisputable except that we could not clarify the cause. Interaction between microorgan-isms found in the digestive tract and the target cells for the toxin has been described in the literature.This has been observed in butterflies .... So thinking in this direction is not erroneous. (....)Have co-factors been included in this type of investigation in the past?No, the effect of co-factors has not really been taken into account. One has to say that this kind ofinvestigation is a huge undertaking. In an investigation of a pesticide it is only the active agent factorthat is examined. Clearly we need to be very careful here and look much more closely at this aspect infuture. Other questions arise too: for instance, is the present testing procedure really adequate in itsdimensions to include co-factors? If we actually find evidence that there is some interaction betweenthe effects of co-factors then we need to get down to work. It is a relatively new view point. (...)
2627
Gen-ethischer Informationsdienst, GID, 194: page 5-9, http://www.gen-ethisches-netzwerk.de/gid/194/kleiner-parasit-grosse-wirkungNosema apsis is a parasite which causes a kind of dysentery in honey bees. It is a microsporidian and is now oftenclassified as fungi.
30 | Risk Reloaded |Interview
In the past you have done other honey bee research. Can you tell us a little bit about this?Before starting the project with the Bt plants we had already done some research on possible hazards tothe health of honey bees due to genetically modified herbicide resistant oil-seed rape and maize plants.We did not find anything negative here. Apart from this we also investigated whether the genes thatcome from the pollen of the plants could be transferred to honey bees. This is called horizontal genetransfer. Our first step was to find out if genes from the plants could be transferred to the microorgan-isms in the digestive tract of the honey bees. Later on we aimed to determine how high the probabilitywas that the honey bees incorporate the genes themselves. One must consider that the crossover ofgenes is one of the principal mechanisms of evolution. It happens in very many groups of organisms.It was more a fundamental question of scientific principles than a practical problem. We cultivated themicroorganisms with the pollen and the result was that the microorganisms had indeed taken up thepat gene.28In the debate on genetic engineering it had always been said that one thing that could neverhappen was the horizontal transfer of newly inserted genes. We presented the results to the Naturejournal and got two expert opinions. One was very positive, thinking it could be published immediately.The other thought we should do an additional analysis, a so-called Southern blot which would furtherverify our results. Then he would back publication. We said, “We’ll do that.” We did the Southern blotand submitted the article again in the belief that there was now nothing in our way. For a long time weheard nothing at all from the editorial team at Nature but in the meantime we were visited by a ZDF(German public television channel) team who asked us about our research. At the time we told themthat nothing could be broadcast until an agreement had been reached with Nature and the article hadbeen published. They nevertheless did broadcast a television programme. It was even on the news – allbefore we had had a final decision fromNature.We intervened strongly whereupon one of the ZDF teamsaid, “Wait a minute, don’t you know that your article has been rejected.” Until that moment we had hadno idea. When we asked him how he knew he said that he had spoken to some people at Monsanto andthey had told him. Naturally I was shocked. It is good that they get to know these things, but I find itawful that they should know before the authors know.How extraordinary!Well, you know that when the person making the decision has contacts to Monsanto says something ...good. But the editorial team – since they were the only ones to have had both reports - that they passthis on, I find that very annoying. Such a highly respected journal. They shouldn’t need to do that. Infact such a review process should first and foremost be.....(falters)....discreet?....very discreet.You probably don’t know the names of either of these editors, do you?No.Do they know your name?Yes, they get the paper and then of course they know the names of the authors. It is not anonymous.Unless you insist. Sometimes that happens. In sensitive cases. I didn’t think our data was so sensitive.We have repeated the experiment. And we have been able to prove that horizontal transfer occurs witha whole series of microorganisms of different kinds.(....)Were your findings published somewhere else later on?No, not yet. Since they are something no one wants to hear it is difficult to find an adequate place forthem. (....)
28
The pat gene in genetically engineered plants mediates a tolerance to the herbicide agent containing glufosinate
Political responsibilities| Risk Reloaded | 31
Political responsibilitiesThe European Union has fixed high standards in its regulations for protecting consumers and theenvironment (Regulation 178/2002, Directive 2001/18, Regulation 1829/2003). A majority of EU memberstates currently seems to be convinced that these standards are not met by the practice for authoris-ing transgenic plants. Since the EFSA started its work, none of its opinions about risk assessments ofgenetically engineered plants has been accepted by the necessary majority of EU member states. Onthecontrary, a majority has even voted against the EFSA’s opinions in several cases.In 2009 the EU Commission came up with a statement that it is no longer going to authorise transgenicplants without having the support of the EU member states. The reason for this announcement is agenetically engineered BASF company potato called Amflora. The EFSA and EU Commission alreadydeclared their consent to its authorisation but no majority was reached in the EU Council. The EU Com-mission has in similar situations so far not hesitated to grant market authorisation. But this was not thecase with Amflora; instead the Commission explained it was not going to proceed with the case if themember states opposed the authorisation.29Unlike in the US, in the EU there is a legal distinction between the institutions that conduct scientificrisk assessment (the responsibility of the EFSA) and those which decide about the overall risk analysesand risk management. These decisions are taken at the political level. Thus the European Commis-sion and EU Council of member states are the ones to decide about market authorisation (see alsoThen&Lorch, 2008b). The connections between risk assessment (EFSA), risk communication (EFSA) andrisk management and risk analysis (political decision) can be summarised as shown in Figure 2:Fig. 2 : “Risk assessment policies”30The EFSA’s work is confinedto scientific risk assessment.The basic requirements of riskassessment policy, on the otherhand, are matters for politicaldecision (by the EU Commissionor EU member states), as is thesubsequent risk management onwhich the decision on a product’sauthorisation made.
5.
In taking its decision on market authorisation, the EU Commission not only has to refer to scientificfindings, it should also take into account ethical and socio-economical considerations. The precaution-ary principle has above all a high priority. Regulation 178/2002 defines risk analysis and risk manage-ment as follows:“‘risk analysis’ means a process consisting of three interconnected components: risk assessment, riskmanagement and risk communication;‘risk assessment’ means a scientifically based process consisting of four steps: hazard identification,hazard characterisation, exposure assessment and risk characterisation;”(Regulation 178/2002, Art.3.10-11).29TAZ, 19.6.2009
30
aus: JRC, 2008
32 | Risk Reloaded |Political responsibilities
On the specific balance between risk assessment and risk management it is stated:“It is recognised that scientific risk assessment alone cannot, in some cases, provide all the informa-tion on which a risk management decision should be based, and that other factors relevant to thematter under consideration should legitimately be taken into account including societal, economic,traditional, ethical and environmental factors and the feasibility of controls.”(Regulation 178/2002,recital 19)In the light of this general framework and taking into account the details as presented in the partsabove some recommendations are made for the EU’s risk analyses as follows.
Some recommendations| Risk Reloaded | 33
Some recommendations(1) Risk assessment of genetically engineered plants should be conducted without preconditions such asassumptions of similarity (familiarity, substantial equivalence) between transgenic plants and plantsderived from conventional breeding. The comparison between genetically engineered and convention-ally bred plants is an essential tool in risk assessment, but substantial equivalence and familiarity can-not serve as a basic approach or starting point. Transgenic plants have to be seen as technically derivedproducts with specific risks and have to be subjected to comprehensive risk assessment per se.(2) Before the applications for market authorisation are filed, a mandatory step by step procedure withsufficiently defined criteria should be introduced. To avoid unnecessary feeding trials and field trials,testing in contained systems should be given more weight. This new step by step procedure could partof an integrated risk analysis which requires closer cooperation between EU member states, the EUCommission and EFSA and also encompasses ethical, socio-economical and risk related issues (see forexample Haslberger, 2006; Gesche &Haslberger, 2006).Possible tests which should be performed in an early step of risk analysis are stress exposures oftransgenic plants under defined conditions (crash tests), profiling metabolic compounds under differentstages of plant growth and environmental conditions, simulations of different ecological systems andinteractions with different external factors. Before feeding and/or field trials take place, socio- economicand ethical questions should be assessed according to defined criteria. Table 3 tries to give an overviewof the early stages of a new step by step procedure for risk analysis.These investigations result in data which allow a first insight into the stability and predictability of thetransgenic plants as well as the formulation of initial hypotheses on the possible effects on particularorganisms and and ecological systems. Investigations like field and feeding trials cannot be fullyreplaced by these early step in the risk analysis. To avoid unnecessary trials which are of ethicaland ecological concern, it is important to perform the testing as proposed in Table 3 in the frameworkof an integrated risk analysis which also takes socio-economic and ethical criteria into account as aprerequisite for further investigations. These requirements of integrated risk analysis should become amandatory part of market authorisation procedures.(3) To support independent risk research, unrestricted access to research material has to be provided.This access has to be guaranteed at the filing date of applications for experimental field trials at thelatest. In addition companies should pay at fixed rates into a publicly organised fund which can be usedas financial resources for independent research projects. The spending of these funds has to be organ-ised in an independent and transparent manner and involve a range of different research groups.(4) Clearly defined rules for the selection of potential staff members of the authorities and other expertsinvolved and their code of conduct have to be developed, as do mechanisms that serve to provide abso-lute transparency about their contacts with industry, to foster the independence of EU authorities.(5) A system that enables effective monitoring of potential health effects at consumer level is a prereq-uisite for any authorisation is. Regarding risks to the environment, case-specific monitoring is neces-sary in any case as soon as a risk potential can be scientifically described. This always should be thecase, for example, if plants produce toxic compounds such as Bt insecticides. Further, in view of thegeneral risks from genetically engineered plants, there are good reasons to apply case specific monitor-ing as a rule, even if just to confirm that no risks can be identified. The existing networks and proposedsystems of ‘general surveillance’ are not sufficient to examine risk assessment findings after marketauthorisation has been given.
6.
34 | Risk Reloaded |Some recommendations
Table 3: Step by step procedure for early stages of integrated risk analysis of genetically engineered plants
Some recommendations| Risk Reloaded | 35
(6) Plants that contain stacked events have to be subjected to a comprehensive examination as completenew applications even if the different events have already passed risk assessment. If new transgenicplants are authorised, interactions with other genetically engineered plants already on the market haveto be assessed.Even if substantially improved concepts for risk assessment apply, it cannot be expected that the risksfrom genetically engineered plants can be controlled or excluded. This dilemma should be communi-cated quite openly. The technical manipulation of the plants’ genome might no longer be seen as a bigtechnical challenge - but insight into the complexity and possible impacts has increased substantiallyin the last few years. It is already evident that even today it is a matter of open discussion whetherthere is a generally authoritative definition of what a gene is (Pearson, 2006). Perceptions and defitionswill differ – depending on the context.The increasing knowledge about the complexity of genome regulation is a strong argument for promot-ing conventional breeding concepts such as marker assisted selection. These new concepts in con-ventional breeding apply recent findings in molecular biology but not invasive methods for technicalmanipulation of genetic information. For the future of plant breeding the usage of the whole rangeof existing biodiversity will be much more relevant than the methods of transferring isolated genesequences. Besides unanswered questions about the true risk from transgenic plants, success in recentconventional breeding also provides strong arguments for a change of paradigm which has been on itsway for some years already – even in some of the bigger seed companies.The shift of paradigm can be traced to patent applications by companies such as Monsanto. As iswritten in its patent application WO2004053055, which claims unintended effects (!) in geneticallyengineered plants:“Nonetheless, the frequency of success of enhancing the transgenic plant is low due to a number of fac-tors including the low predictability of the effects of a specific gene on the plant’s growth, developmentand environmental response, the low frequency of maize transformation, the lack of highly predictablecontrol of the gene once introduced into the genome, and other undesirable effects of the transformationevent and tissue culture process.”All in all it is time for a fundamental rethink of risk assessment and usage of genetically engineeredplants in agriculture and food production.
36 | Risk Reloaded |References
ReferencesAbel, C. A., Adamczyk, J. J. (2004) Expression of Cry1A in maize leaves and cotton bolls with diverse chlorophyll content and cor-responding larval development of fall armyworm (Lepidoptera: Noctuldae) and southwestern corn borer (Lepidoptera: Crambidae)on maize whorle leaf profiles. Journal of Economic Entomology 97: 1737-1744.Accinelli, C., Serepanti, C., Vicari, A. & Catizone, P. (2004) Influence of insecticidal toxins from Bacillus thuringiensis subsp.kurstaki on the degredation of glyphosate and glufosinate-ammonium in soil samples. Agriculture, Ecosystems and Environment,103: 497-507Anholt, R.R. et al. (2003) The genetic architecture of odor-guided behavior in Drosophila, Nature Genetics 35, 180-184Benachour, N., Séralini, G.-E. (2008) glyphosate Formulations Induce Apoptosis and Necrosis in Human Umbilical, Embryonic, andPlacental Cells, Chem. Res. Toxicol., DOI: 10.1021/tx800218nBøhn T., Primicerio R., Hessen D.O., Traavik T. (2008) Reduced fitness of Daphnia magna fed a Bt-transgenic maize variety: ArchEnviron Contam Toxicol 55(4):584-92Broderick N.A., Raffa K.F., Handelsman J. (2006) Midgut bacteria required for Bacillus thuringiensis insecticidal activity: PNAS103(41): 15196-15199Broderick N.A., Robinson C.J., McMahon M.D., Holt J., Handelsman J., Raffa K.F. (2009) Contributions of gut bacteria to Bacillusturingiensis - induced mortality vary across a range of Lepidoptera: BMC Biology 7: 11Bruns, H. A., Abel, C. A. (2007) Effects of nitrogen fertility on Bt endotoxin levels in maize. Journal of Entomological Science, 42:35-43.Chen, D. Ye, G., Yang, C., Chen Y., Wu, Y. (2005) The effect of high temperature on the insecticidal properties of Bt Cotton. Environ-mental and Experimental Botany 53: 333–342Clark, R.M., Schweikert, G., Toomajian, C., Ossowski, S., Zeller,G., Shinn, P., Warthmann, N., Hu, T. T., Fu G., Hinds, D.A., Chen H.,Frazer, K.A., Huson, D.H., Schölkopf, B., Nordborg, M., Rätsch, G., Ecker, J.R., Weigel D. (2007) Common Sequence PolymorphismsShaping Genetic Diversity in Arabidopsis thaliana, Science, Vol. 317. no. 5836: 338 - 342Codex Alimentarius (2003) Guideline for the conduct of food safety assessment of foods derived from recombinant- DNA plants,CAC/GL 45: 15-18. Rome: Codex Alimentarius Commission. http://www.codexalimentarius.net/web/more_info.jsp?id_sta=10021Coghlan, A., (1999) Splitting headache, Monsanto’s modified soybeans are cracking up in the heat. New Scientist, 20th November:25.Commission of the European Communities (2000) White Paper on Food Safety, COM (1999) 719 final , http://ec.europa.eu/food/food/intro/white_paper_en.htmCotter, J. & Mueller, W. (2009) A critique of the European Food Safety Authority’s opinion on genetically modified maize MON810.Greenpeace Research Laboratories Technical Note 05/2009. Jointly commissioned by Greenpeace and Friends of the Earth Europe.www.greenpeace.org/eu-unit/press-centre/reports/review-EFSA-MON810-opinion-29-07-09Dalton, R. (2008) Modified genes spread to local maize. Nature, Jg. 456, 7219 : 149 doi:10.1038/456149aDiehn, S.H., De Rocher, E.J., Green, P.J. (1996) Problems that can limit the expression of foreign genes in plants: Lessons to belearned from B.t. toxin genes. Genetic Engineering, Principles and Methods 18: 83-99
References| Risk Reloaded | 37
EFSA (2009) Scientific Opinion of the Panel on Genetically Modified Organisms on applications (EFSA-GMO- RX-MON810) for therenewal of authorisation for the continued marketing of (1) existing food and food ingredients produced from genetically modifiedinsect resistant maize MON810; (2) feed consisting of and/or containing maize MON810, including the use of seed for cultivation;and of (3) food and feed additives, and feed materials produced from maize MON810, all under Regulation (EC) No 1829/2003 fromMonsanto. The EFSA Journal (2009) 1149: 1-84. http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902628240.htmEFSA (2008) Statement of the Scientific Panel on Genetically Modified Organisms in response to the request of the European Com-mission on the need for a 90 day rodent feeding study with genetically modified rice LLRICE62 (Question N� EFSA-Q-2008-342)Adopted on 2nd July 2008 ,http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902011178.htm Safety and NutritionalEFSA (2007a) Assessment of Genetically Modified Plants and Derived Food and Feed: The Role of Animal Feeding Trials – Reportof the EFSA GMO Panel working group on animal feeding trials: Adopted by the Scientific panel on Genetically Modified Organ-isms on 12 September 2007. Food and Chemical Toxicology, Volume 46, Supplement 1, March 2008, http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902590265.htmEFSA (2007b), Guidance Document of the Scientific Panel on Genetically Modified Organisms for the risk assessment of geneticallymodified plants containing stacked transformation events, The EFSA Journal (2007) 512, 1-5, http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902599859.htmEFSA (2006) Guidance document for the risk assessment of genetically modified plants and derived food and feed by the ScientificPanel on Genetically Modified Organisms (GMO) - including draft document updated in 2008. The EFSA Journal 727: 1-135; draftdocument adopted in May 2008. http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1211902599947.htmEFSA (2005a) Opinion of the Scientific Panel on Genetically Modified Organisms on a request from the Commission related to thenotification (Reference C/F/96/05.10) for the placing on the market of insect resistant genetically modified maize Bt11, for cultiva-tion, feed and industrial processing, under Part C of Directive 2001/18/EC from Syngenta Seeds. The EFSA Journal (2005) 213,1-33.EFSA (2005b) Opinion of the Scientific Panel on Genetically Modified Organisms on a request from the Commission related to thenotification (Reference C/ES/01/01) for the placing on the market of insect-tolerant genetically modified maize 1507 for import,feed and industrial processing and cultivation, under Part C of Directive 2001/18/EC from Pioneer Hi-Bred International/MycogenSeeds. The EFSA Journal (2005) 181, 1-33.ENCODE 2007, “Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project”, Na-ture, Vol 447, 14 Juni 2007, 799 – 816.European Communities (2005) Measures affecting the approval and marketing of biotech products (DS291, DS292, DS293). Com-ments by the European Communities on the scientific and technical advice to the panel. 28 January 2005, http://trade.ec.europa.eu/doclib/html/128390.htmEwen S., Pusztai, A. (1999) Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat smallintestine“, The Lancet, Vol. 354: 1353-1354FAO/WHO (2000) Safety aspects of genetically modified foods of plant origin. Report of a joint FAO/WHO expert consultation onfoods derived from biotechnology, 29 WHO/FAO, 2000, http://www.fao.org/ag/agn/food/pdf/gmreport.pdfFernandez-Martinez J.M et al., (1997) Sunflower mutant containing high levels of palmitic acid in high oleic background, Euphytica97:113-116.Finamore, A., Roselli, M., Britti, S., Monastra, G., Ambra, R., Turrini A., Mengheri, E. (2008) Intestinal and Peripheral Immune Re-sponse to MON810 Maize Ingestion in Weaning and Old Mice, J. Agric. Food Chem., 2008, 56 (23): 11533–11539
38 | Risk Reloaded |References
Finnegan H. (1994) Transgene Inactivation, plants fight back, Bio/Technology12:883-888Gasnier, C., Dumont, C., Benachour, N., Clair, E., Chagnon, M.C., Seralini G.E. (2009) glyphosate-based herbicides are toxic andendocrine disruptors in human cell lines, Toxicology, http://dx.doi.org/10.1016%2Fj.tox.2009.06.006Gertz J.M., Vencill W.K., Hill N.S. (1999) Tolerance of Transgenic Soybean (Glycine max) to Heat Stress. British Crop ProtectionConference – Weeds, 15-19 Nov 1999,. Brighton: 835-840.Gesche, A., Haslberger, A., (2006) Governing sustainable food and farming production futures using integrated risk assessmentapproaches. In Kaiser, M and Lien, M.E., Eds. Proceedings Ethics and the politics of food; Congress of the European Society forAgricultural and Food Ethics, pages pp. 402-407, Oslo. Accessed from http://eprints.qut.edu.auGrant-Downton R.T., Dickinson H.G. (2005) Epigenetics and its Implications for Plant Biology. 1. The Epigenetic Network in Plants.Ann. Bot. 96: 1143-1164.Risk Underestimated, Interviews with nine scientists on the subject of genetically modified plants http://www.greenpeace.de/themen/gentechnik/nachrichten/artikel/gen_pflanzen_das_unterschaetzte_risiko/Griffiths, B. S., Caul, S., Thompson, J., Birch, A. N., Scrimgeour, C., Cortet, J., Foggo, A., Hacket, C. A., Krogh, P. H. (2006) Soil micro-bial and faunal community responses to Bt maize and insecticide in two soils. Journal of Environmental Quality, 35: 734-741.Haslberger A.G. (2006). Need for an “Integrated Safety Assessment” of GMOs, Linking Food Safety and Environmental Consider-ations. J Agric Food Chem: 3173-3180Hilbeck A, Schmidt JEU (2006) Another view on Bt proteins - How specific are they and what else might they do?: BiopesticidesInternational 2(1): 1-50JRC (2008) Joint Research Centre – Institute for Prospective Technological Studies, European Commission, Risk-assessmentpolicies: Differences across jurisdictions, Authors: Erik Millstone, Patrick van Zwanenberg, Les Levidow, Armin Spök, HideyukiHirakawa, Makiko Matsuo , Scientific and Technical Research series – ISSN 1018-5593, EUR 23259 ENJRC (2009) Joint Research Centre – Institute for Prospective Technological Studies, European Commission, The global pipeline ofnew GM crops Implications of asynchronous approval for international trade, authors: Alexander J. Stein and Emilio Rodríguez-Cerezo EUR 23486 ENKaatz HH (2005) Auswirkungen von Bt- Maispollen auf die Honigbiene, Uni Jena, Sicherheitsforschung und Monitoring zum Anbauvon Bt-Mais. http://www.biosicherheit.de/de/sicherheitsforschung/68.doku.htmlKramarz PE, Vaufleury A, Zygmunt PMS, Verdun C (2007) Increased response to cadmium and bacillus thuringiensis maize toxic-ity in the snail Helix aspersa infected by the nematode Phasmarhabditis hermaphrodita: Environmental Toxicology and Chemis-try 26 (1): 73–79Kroghsbo, S., Madsen, C., Poulsen M. et al. (2008) Immunotoxicological studies of genetically modified rice expressing PHA-E lectinor Bt toxin in Wistar rats. Toxicology, 245: 24-34.Kuiper H.A., Kleter G.A., Noteborn, H.P.J.M, Kok, E.J. (2001) Assessment of the food safety issues related to genetically modifiedfoods. Plant J. 27 (6): 503-528)Li H, Buschman LL, Huang F, Zhu KY, Bonning B, Oppert BA (2007) Resistance to Bacillus thuringiensis endotoxins in the Euro-pean corn borer: Biopestic. Int. 3 (2): 96-107.Lorch, A. (2005) Bt11- C/F/96.05.10, Bt Maize notification for cultivation, report prepared on behalf of Greenpeace International,http://www.saveourseeds.org/dossier/dossier_bt11_1507.html
References| Risk Reloaded | 39
Lorch, A. & Then, C. (2007) How much Bt toxin do genetically engineered MON810 maize plants actually produce? Bt concentrationin field plants from Germany and Spain. Greenpeace e.V., (http://www.greenpeace.de/publikationen/)Lorch A.& Then, C. (2008) Kontrolle oder Kollaboration, Studie im Auftrag von Uli Höfken, www.ulrike-hoefken.de/cms/default/dokbin/232/232887.kontrolle_oder_kollaboration_agrogentech.pdfMalatesta M, Caporaloni C, Gavaudan S, Rocchi MBL, Tiberi C, Gazzanelli G. (2002) Ultrastructural morphometrical and immuno-cyto-chemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean. Cell Struct Funct 2002a; 27:173-80.Malatesta, M.Biggiogera M, Manuali F, Rocchi MB, Baldelli B, Gazzanelli G. (2003) Fine structural analyses of pancreatic acinarcell nuclei from mice fed on genetically modified soybean. Eur J Histochem 47:385–388Malatesta,M, Tiberi C., Baldelli B., Battistelli,S., Manuali, E., Biggiogera M. (2005) Reversibility of hepatocyte nuclear modificationsin mice fed on genetically modified soybean , Eur. J. Histochem, Vol 49 (3): 237-242Matthews D, Jones H, Gans P, Coates St & Smith LMJ (2005) Toxic secondary metabolite production in genetically modified potatoesin response to stress. Journal of Agricultural and Food Chemistry, 10.1021/jf050589r.Mattick, J.S. (2003) Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms, BioEssays 25:930-939Mertens, M. (2008) RoundupReady Sojabohne – Wiederzulassung in der EU? Gutachtenerstellt im Auftrag des Bund für Umwelt und Naturschutz Deutschland e.V. und Friends ofthe Earth Europe. Online verfügbar unter http://www.gentechnikfreie-regionen.de/fileadmin/content/studien/risikobewertung/SojaRRRoundupReady_SojabohneKfinal.pdf,Meyer, H.H.D. et al. (2009) Abschlußbericht zum Forschungsvorhaben A/05/12 , „Einsatz von transgenem Mais (MON810) beiMilchkühen: Abbau, Transfer sowie potentielle Interaktionen von DNA und Bt-Protein im Rind“ , TU München, Bayerische Lande-sanstalt für Landwirtschaft (LfL) und Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt. www.lfl.bayern.de/ite/rind/35021/linkurl_0_2_0_0.pdfMoch, Katja (2006) Epigenetische Effekte bei transgenen Pflanzen: Auswirkungen auf die Riskobewertung, BfN-Skripten 187,Bundesamt für Naturschutz, Bonn, http://www.bfn.de/0502_gentechnik.html?no_cache=1Myhre, M.R., Fenton,K.A., Eggert, J., Nielsen K.M. and Traavik, T. (2006) The 35S CaMV plant virus promoter is active in humanenterocyte-like cells, European Food Research and Technology, Volume 222, Numbers 1-2, 185-193Nguyen, H. T., Jehle, J. A. (2007) Quantitative analysis of the seasonal and tissue-specific expression of Cry1Ab in transgenic maizeMon810. Journal of Plant Diseases and Protection 114: 82.Nijhout, H.F. (2003) The importance of context in genetics. American Scientist 91: 416-423OECD Consensus Documents for the work on the Safety of Novel Foods and Feeds, OECD, http://www.oecd.org/document/9/0,2340,en_2649_34391_1812041_1_1_1_37437,00.htmlOECD (1993) Safety Considerations for Biotechnology: Scale-up of Crop Plants. OECD, 1993, http://www.oecd.org/datao-ecd/26/26/1958527.pdf?channelId=34537&homeChannelId=33703&fileTitle=Safety+Considerations+for+Biotechnology+Scale-up+of+Crop+PlantsPearson,H. (2006), What is a Gene?, Nature 441: 399-401Pickardt, T. (2002) Stabilität transgen-vermittelter Merkmale in gentechnisch veränderten Pflanzen.mit dem Schwerpunkt trans-
40 | Risk Reloaded |References
gene Gehölzarten und Sterilitätsgene, Studie im Auftrag des Bundesumweltamtes UBA, Texte 53/02, Forschungsbericht 20167430/2Pigott CR, Ellar DJ (2007) Role of Receptors in Bacillus thuringiensis Crystal Toxin Activity: Microbiol Mol Biol Rev 71 (2): 255–281Prescott V.E., Campbell P.M., Moore A., Mattes J., Rothenberg M.E., Foster P.S., Higgins T.J., Hogan S.P. (2005) Transgenic expressionof bean alpha-amylase inhibitor in peas results in altered structure and immunogenicity. J. Agric. Food Chem. 53: 9023-9030.Quist, D. & Chapela I.H. (2001) Transgenic DNA introgressed into traditional maize landraces in Oaxaca, Mexico, Nature volume414: 541-543Rang, A., Linke, B., Jansen, B. (2004) Detection of RNA variants transcribed from the transgene in Roundup Ready soybean, EurFood Res Technol (2005) 220:438–443Rosi-Marshall E.J., Tank J.L., Royer T.V., Whiles M.R., Evans-White M., Chambers C., Griffiths N.A., Pokelsek J., Stephen M.L. (2007)Toxins in transgenic crop by-products may affect headwater stream ecosystems, Proceedings of the National Academy of Sciences104: 16204 – 16208Rosati, A., Bogani, P., Santarlasci, L., Buiatti, M. (2008) Characterisation of 3’ transgene insertion site and derived mRNAs inMON810 YieldGard maize. Plant Molecular Biology, 67: 271-281.Rowe H.C., Hansen B.G., Halkier B.A. & Kliebenstein D.J. (2008) Biochemical networks andepistasis shape the Arabidopsis thaliana metabolome. Plant Cell 20, 1199-1216.Sagstad, A., Sanden, M., Haugland, Ø., Hansen, A.-C., Olsvik, P.A., Hemre, G.-I. (2007) Evaluation of stress- and immune-responsebiomarksers in Atlantic salmon, Salmon salar L., fed different levels of genetically modified maize (Bt maize), compared with itsnear- isogenic parental line and a commercial suprex maize. Journal of Fish Disease, 30: 201- 212.Sauter , A. (2008) Transgenes Saatgut in Entwicklungsländern – Erfahrungen, Herausforderungen, Perspektiven, Endbericht zumTA-Projekt „Auswirkungen des Einsatzes transgenen Saatguts auf die wirtschaftlichen, gesellschaftlichen und politischen Struk-turen in Entwicklungsländern“, Büro für Technikfolgenabschätzung beim Deutschen Bundestag, Arbeitsbericht Nr. 128 www.tab.fzk.de/de/projekt/zusammenfassung/ab128.pdfSchmidt J.E.U, Braun C.U., Whitehouse L.P., Hilbeck A. (2008), Effects of activated Bt transgene products (Cry1Ab, Cry3Bb) onimmature stages of the ladybird Adalia bipunctata in laboratory ecotoxicity testing: Arch Environ Contam Toxicol, DOI 10.1007/s00244-008-9191-9Schnepf E., Crickmore N., van Rie J., Lereclus D., Baum J., Feitelson J., Zeigler D.R., Dean D.H. (1998), Bacillus thuringiensis and itspesticidal crystal proteins: Microbiol Mol Biol Rev. 62(3): 775-806Séralini G.E., Vendômois, J.S., Cellier, D.,Sultan C., Buiatti, M., Gallagher, L. Antoniou, M., .Dronamraju, K.R. (2009) How Subchronicand Chronic Health Effects can be Neglected for GMOs, Pesticides or Chemicals, Int. J. Biol. Sci., 5(5):438-443Séralini G.-E., Cellier D. & Spiroux de Vendomois J. (2007) New analysis of a rat feeding study with a genetically modified maizereveals signs of hepatorenal toxicity. Archives of Environmental Contamination and Toxicology 52, 596-602.Service, R. F. (2007) A growing threat down on the farm. In: Science, Jg. 316, S. 1114–1117.Schiefer, Ch., Schubert R., Pölitz, B., Kühne, A., Westphal, K., Steinhöfel, O., Schaerff, A., (2008) Untersuchungen zu Konsequenzendes Anbaus von GVO in Sachsen, Schriftenreihe der Sächsischen Landesanstalt für Landwirtschaft, Heft 15/2008Spök A, Hofer H, Lehner P, Valenta R, Stirn S, Gaugitsch H (2004): Risk Assessment of GMO Products in the European Union. Toxic-
References| Risk Reloaded | 41
ity assessment, allergenicity assessment and substantial equivalence in practice and proposals for improvement and standardisa-tion: Vienna: Umweltbundesamt, 2004 Reports Series vol. 253, http://www.bmgf.gv.at/cms/site/attachments/6/8/7/CH0255/CMS1090828056047/risk_assessment_of_gmo_products-bmgf-layout.pdf.Spök, A., Eckerstorfer, M., Heissenberger, A., Gaugitsch, H. (2007) Risk Assessment of “stacked events” Forschungsberichte derSektion IV Band 2/2007, http://www.bmgfj.gv.at/cms/site/standard.html?channel=CH0810&doc=CMS1180523433481Steimer A, Schöb H, Grossniklaus U (2004): Epigenetic control of plant development: new layers of complexity. Curr. Opin. PlantBiotech. 7: 11-19.Steinke K., Paul V., Gürtler P., Preißinger W., Wiedemann S., Albrecht C., Spiekers H., Meyer H.H.D., Schwarz F.J. (2009) Effectsof long-term feeding of genetically modified maize (Bt-maize, MON810) to dairy cows on performance and metabolic parameters.Proceedings of the Society of Nutrition Physiology 18 (2009) 110Tabashnik, B.E., Liu, Y.-B., Finson, N., Masson, L., Heckel, D.G. (1997) One gene in diamondback moth confers resistance to fourBacillus thuringiensis toxins, Proc. Natl. Acad. Sci. USA, Vol. 94, pp. 1640–1644Tabashnik, B. E., Unnithan, G.C., Masson, L., Crowder, D.W., Li, X., Carriere, Y. (2009) Asymmetrical cross-resistance between Bacil-lus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm, Proc. Natl Acad. Sci. USA advance online publication doi:10.1073/pnas.0901351106Tang, G., Qin, J., Dolnikowski G.G., Russell R.M., Grusak M.A. (2009) Golden Rice is an effective source of vitamin A, Am J ClinNutr 89: 1776-1783,The Encode Project Consortium (2007) Identification and analysis of functional elements in 1% of the human genome by the EN-CODE pilot project“, Nature, Vol 447, 14. Juni 2007, pages 799 bis 816Then, C. (2009a) The campaign for genetically modified rice is at the crossroads A critical look at Golden Rice after nearly 10 yearsof development . foodwatch e.V. http://www.foodwatch.de/english/golden_rice/index_ger.htmlThen C. (2009b) Risk assessment of toxins derived from Bacillus thuringiensis - synergism, efficacy, and selectivity, Environmen-tal Science and Pollution Research, http://dx.doi.org/10.1007/s11356-009-0208-3Then, C. (2009c) Bayerische Fütterungsstudie der TU München zu Gen-Mais weist Mängel auf, on hehalf of Greenpeace Germanye.V., April 2009 http://www.greenpeace.de/fileadmin/gpd/user_upload/themen/gentechnik/Kritische_Stellungnahme_Fuetter-ungsversuch_final_gesamt.pdfThen, C; Brockmann K. (2009), Lässt sich der Anbau von Gen-Mais Mon810 in Deutschland verbieten? Eine wissenschaftliche undrechtliche Bewertung, Bund für Ökologische Lebensmittelwirtschaft und Campact, www.boelw.de/uploads/media/Studie_Ver-bot_MON810_090402_.pdfThen C. & Lorch A. (2008a) A simple question in a complex environment: How much Bt toxin do genetically engineered MON810maize plants actually produce?: in Breckling B, Reuter H, Verhoeven R (eds) (2008) Implications of GM-Crop Cultivation at LargeSpatial Scales., Theorie in der Ökologie 14. Frankfurt, Peter Lang, http://www.gmls.eu/index.php?contact=jaThen, C.; Lorch, A. (2008b) EU-Risikomanagement: Risikobewertung und -management von Lebensmitteln - der Schlingerkurs derEU-Kommission. Herausgegeben von Hiltrud Breyer, http://www.hiltrud- breyer.eu/hbreyer/media/doc/1228483018143.pdfTraavik, T. (2008) GMOs and their unmodified counterparts: substantially equivalent or different? in: Breckling, B., Reuter, H. &Verhoeven, R. (2008) Implications of GM-Crop Cultivation at Large Spatial Scales, Theorie in der Ökologie 14. Frankfurt, PeterLang.
42 | Risk Reloaded |References
Valenta, R. &Spök, A. (2008) Immunogenicity of GM peas, BfN Skripten 239, Bundesamt für Naturschutz, Bonn, http://www.bfn.de/0301_veroe.htmlVecchio L, Cisterna B, Malatesta M, Martin TE, Biggiogera M., (2004) Ultrastructural analysis of testes from mice fed on geneti-cally modified soybean Eur J Histochem, 48:449-53.Velimirov, A., Binter C., Zentek J., (2008) Biological effects of transgenic maize NK603xMON810 fed in long term reproduction stud-ies in mice. Austrian Federal Ministry for Health, Family and Youth Forschungsberichte der Sektion IV, Band 3/2008, http://www.bmgfj.gv.at/cms/site/attachments/3/2/9/CH0810/CMS1226492832306/forschungsbericht_3-2008.pdfVenter, J.C. et al (2001) The sequence of the Human Genome. Science Vol. 291: 1304-1351Wentzell A.M., Boeye I., Zhang Z., Kliebenstein D.J. (2008) Genetic Networks Controlling Structural Outcome of Glucosinolate Acti-vation across Development. PLoS Genet 4(10): e1000234. doi:10.1371/journal.pgen.1000234WHO (1999) High Dose Irradiation: Wholesomeness of food irradiated with doses above 10 kGy, Joint FAO/IAEA/WHO StudyGroup, WHO Technical Report Series 890Wilson A., Lathman J. & Steinbrecher R. (2006): Transformation-induced mutations in transgenic plants: Analysis and biosafetyimplications. Biotechnology and Genetic Engineering Reviews 23, 209-237.Ye, X., Al-Babili, S., Klöti, A., Zhang, J., Lucca, P., Beyer, P., Potrykus, I (2000) Engineering the provitamin A (ß -carotene) biosyn-thetic pathway into (carotenoid-free) rice endosperm, Science, 287, pages 303-305.
Concluding remarks| Risk Reloaded | 43
Concluding remarksTestbiotech e.V. was founded by a group of experts in 2008 and this report is its public debut. TestBio-Tech supports independent research and public debate on the effects of biotechnology. It is an organisa-tion which sees itself as an interface between science and society with a intermediary role between thepublic, research and politics. Its main concerns are the ecological, social and ethical consequences ofbiotechnology, particularly the application of genetic engineering in agriculture. TestBioTech supportsindependent risk research.In recent years the team at Testbiotech have worked on and acquired expertise in various aspects ofmodern biotechnology, some of which has been factored into this report.Christoph Then has amongst other things recently worked on risk assessment for genetically engi-neered plants which produce the Bt insecticide. His contributions have been published in the scientificmedia (Then, 2009b, Then & Lorch, 2008a) and cited in discussions on science and politics by variousnon-governmental organisations (Then 2009a, 2009c, Then & Brockman, 2009). His book Dolly ist tot(“Dolly is Dead”) was published in 2008 by Rotpunktverlag publishers in Zurich.Christof Potthof works for the Gen-ethischen Netzwerk network (www.gen-ethisches-netzwerk.de)and is the editor for the Gen-ethischen Informationsdienst service GID. His contributions focus onthe scientific, political and economic fields of agro-genetic engineering. His interview with ProfessorHans-Hinrich Kaatz appeared in GID 194. It deals with the problems of independent risk research inGermany and appears here in extracts.Our special thanks at this point to the GEKKO foundation and the BonVenture gGmbH, who supportour work and therefore make our independence possible. This report has been financed by the GEKKOfoundation.
TESTBIOTECHTestbiotech e. V.Institute for IndependentImpact Assessmentin Biotechnology
Risk analysis of genetically engineered plants within the European UnionA report by Testbiotech e.V.Institute for Independent Impact Assessment in Biotechnologywww.testbiotech.orgAuthors: Christoph Then, Christof PotthofEditing: Andrea ReicheOctober 2009