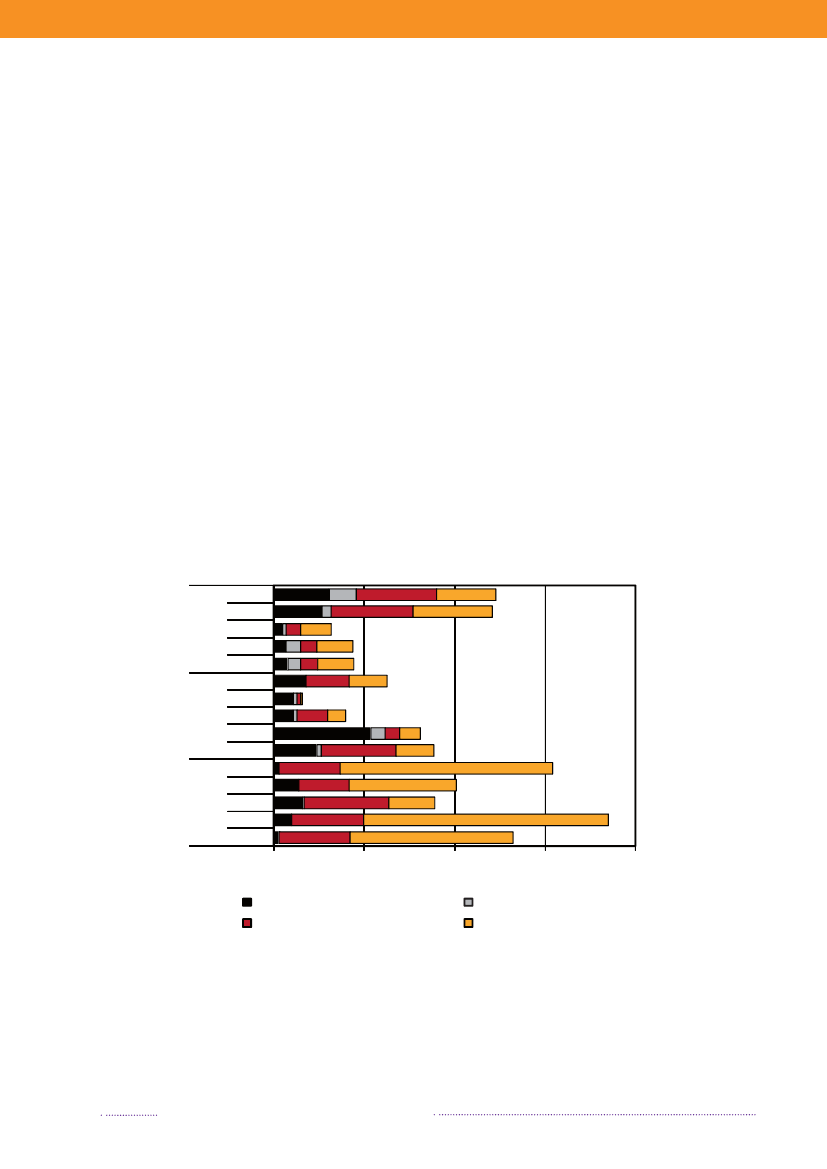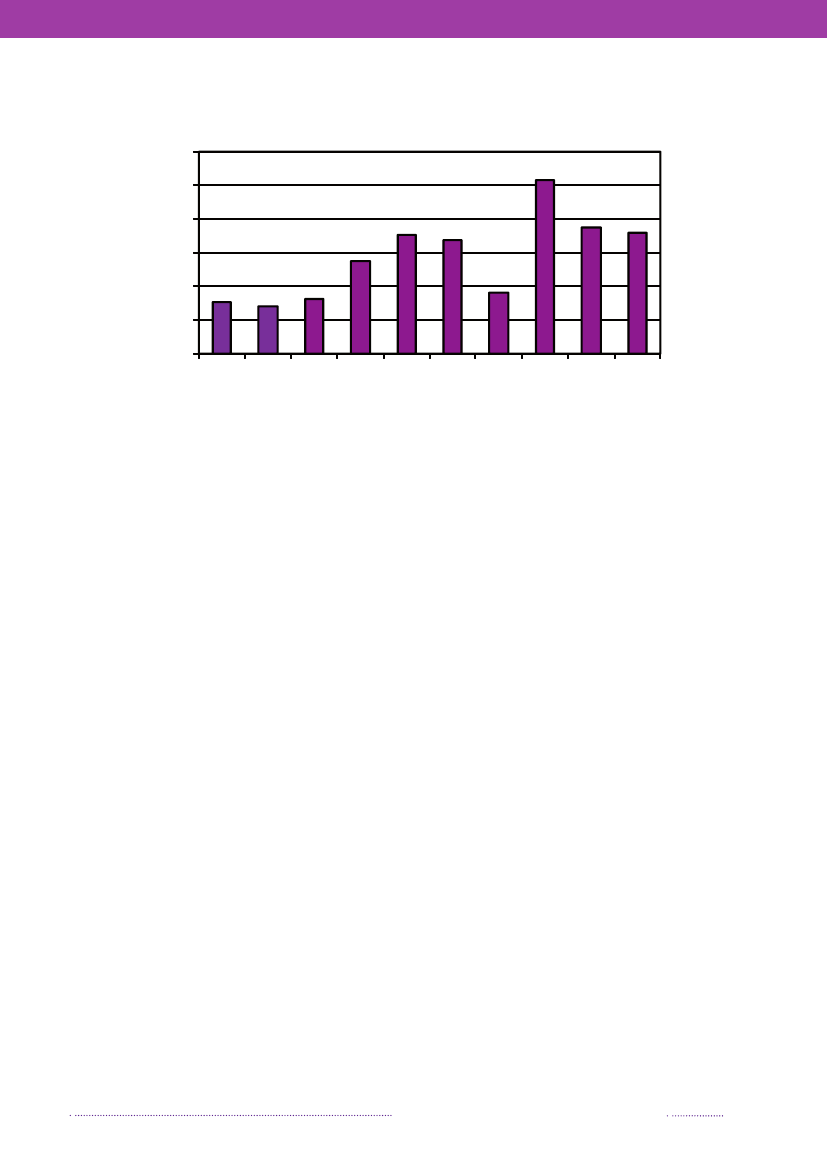Udvalget for Fødevarer, Landbrug og Fiskeri 2009-10
FLF Alm.del Bilag 100
Offentligt
Annual Report on Zoonosesin Denmark 2008
Annual Report on Zoonoses in Denmark 2008Edited by:Birgitte Helwigh and Anne Louise KroghThe Danish Zoonosis CentreNational Food InstituteTechnical University of DenmarkSteen EthelbergStatens Serum InstitutThis is an official publication from theNational Food Institute, TechnicalUniversity of Denmark, the DanishVeterinary and Food Administration andStatens Serum Institut.Text and tables may be cited and reprintedonly with reference to this report.Suggested citation:Anonymous, 2009. Annual Report onZoonoses in Denmark 2008, National FoodInstitute, Technical University of Denmark.Reprints can be ordered from:The Danish Zoonosis CentreNational Food InstituteTechnical University of DenmarkMørkhøj Bygade 19DK - 2860 SøborgDenmarkPhone: +45 35 88 70 00Fax:+45 35 88 70 01E-mail: [email protected]Layout: Susanne CarlssonPhotos: Colourbox and Mikkel AdsbølPrinting: Schultz Grafisk A/SISSN 0909-3837The report is also available at:www.food.dtu.dk
ContentsIntroduction1233.13.23.33.44.14.24.34.31.1 Salmonella Source Account 2008
561012
Trends and sources in human salmonellosisOutbreaks of special interest
Typing methods used in the surveillanceof foodborne pathogensSurveillance of human infectionsSurveillance of food and animal isolatesOutbreak detection and investigationNational and international cooperation
4. VTEC - staus and human risk factors
Human cases in 2008VTEC in cattleVTEC in minced beef meat - 2008 investigationRisk factors for sporadic VTEC infections in Denmark
16
5. EU related topics
5.1 Trichinella special status5.2 Control of Salmonella in animal populations- EU regulations and studies
21
6. Surveillance and monitoring6.16.26.36.4
Surveillance of human diseaseOutbreaks of zoonotic gastrointestinal infectionsSurveillance and monitoring of animals and animal productsOfficial testing of zoonotic pathogens in foodstuffsTrends and sources in human salmonellosisHuman disease and outbreak dataMonitoring and surveillance dataMonitoring and surveillance programmesPopulation and slaughter dataList of Figures and Tables
20
AppendixAppendix A.Appendix B.Appendix C.Appendix D.Appendix E.Appendix F.
242531445052
4
Annual Report on Zoonoses in Denmark 2008
Introduction
Annual Report on Zoonoses presents a summary of the trends and sources of zoonotic infections in humans andanimals and the occurrence of zoonotic agents in food and feeding stuffs in Denmark in 2008. Greenland and the FaeroeIslands are not covered by the report. The report is based on data from the national surveillance and control programmesincluding data collected according to Zoonoses Directive 2003/99/EC, supplemented by data from relevant researchprojects. The report is also available at www.food.dtu.dk.A general description of the surveillance and monitoring of zoonoses is an important part of this report. All surveil-lance programmes and results are presented in appendix tables, and selected results are also presented in a narrative form.This years report contains focused chapters on the typing methods used for surveillance and outbreak detection andon Verocytotoxin-producingEscherichia coliin Denmark. In addition, the 2008Salmonellasource account and descrip-tions of the most interesting outbreaks are presented in separate chapters. Please note that corrections to the data mayoccur after publication, resulting in minor changes in the presentation of historical data in the following years reports.
Profile of the yearIn 2008, there were severalSalmonellaTyphimurium outbreaks and, in particular, one very largeS.Typhimuriumphage type U292 outbreak with 1,224 registered cases in 2008 was the subject of an intensive investigation, but thesource of the outbreak remained unknown. The outbreak is the largest knownSalmonellaoutbreak in Denmark todate. Other relatively large outbreaks caused byS.Typhimurium strains belonging to the phage types DT135, DT120and U288 also occurred.The number of humanSalmonellacases estimated to be due to travelling was at the same level as in 2007 (the firstyear with active surveillance for infections acquired abroad), however the number of cases attributed to Danish porkincreased three-fold mainly due to the large outbreaks.Infections with verocytotoxin-producingE. coliare of particular concern because of their relative seriousness. Ap-proximately half of the country is covered by molecular diagnostics; at 2.9 cases per 100.000 population, the incidencewas at the same level as in recent years. A case-control study of risk factors for sporadic infections indicated strawberriesand contaminated public water supply as risk factors.
Annual Report on Zoonoses in Denmark 2008
5
1. Trends and sources in humansalmonellosisBy Sara Monteiro Pires ([email protected]) andTine Hald
1.1Salmonellasource account 2008The Danish Zoonosis Centre routinely applies a so-cal-led ”source attribution” model to estimate the contributionof the major animal-food sources to human infections ofSalmonella.The principle of the method is to compare thenumber of human cases caused by differentSalmonellasero- and phage types with the distribution of the samesubtypes isolated from the various animal-food sources.Antimicrobial resistance profiles ofS.Typhimurium isola-tes are also included to further distinguish between similarphage types found in animals, food and humans. Since themodel was first implemented in 1995, it has evolved frombeing purely deterministic to becoming a stochastic model,built under a Bayesian framework. In 2008, a new metho-dological development was introduced in the model (1),which applies data from multiple years thereby improvingthe robustness and accuracy of the results without compro-mising their comparability with estimates from previous
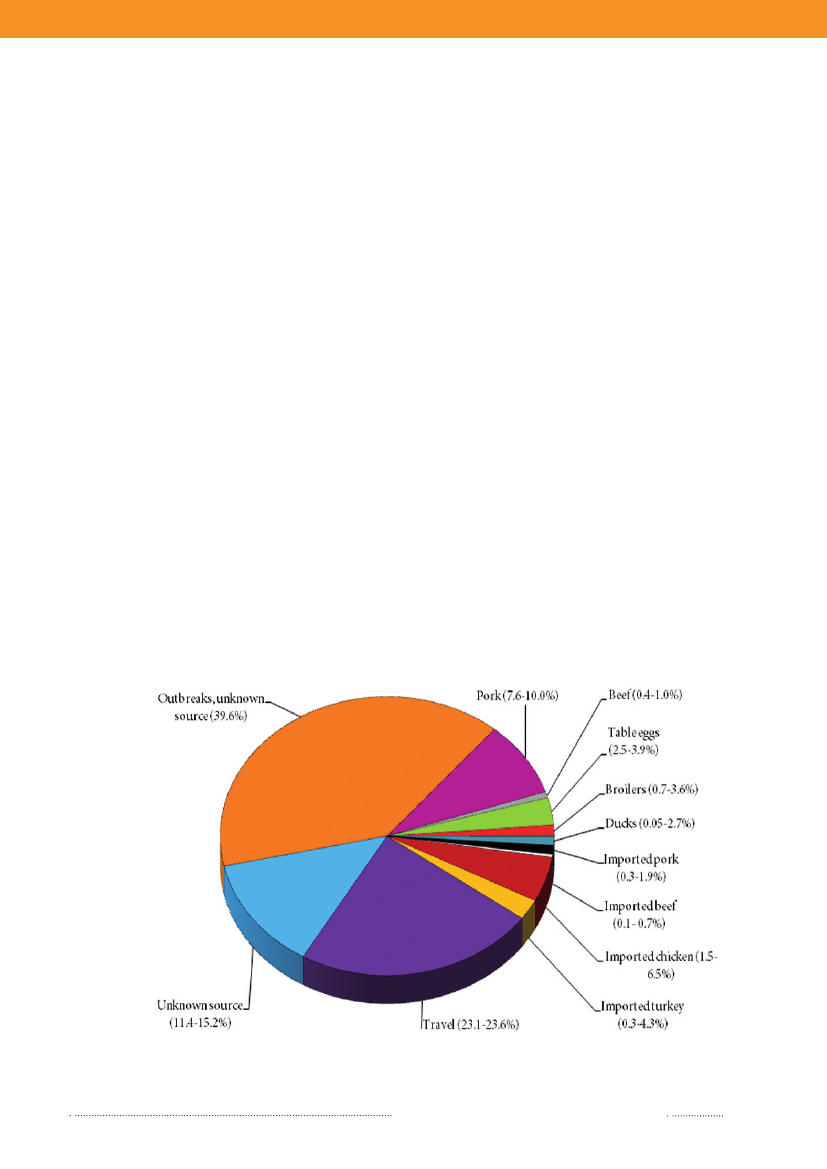
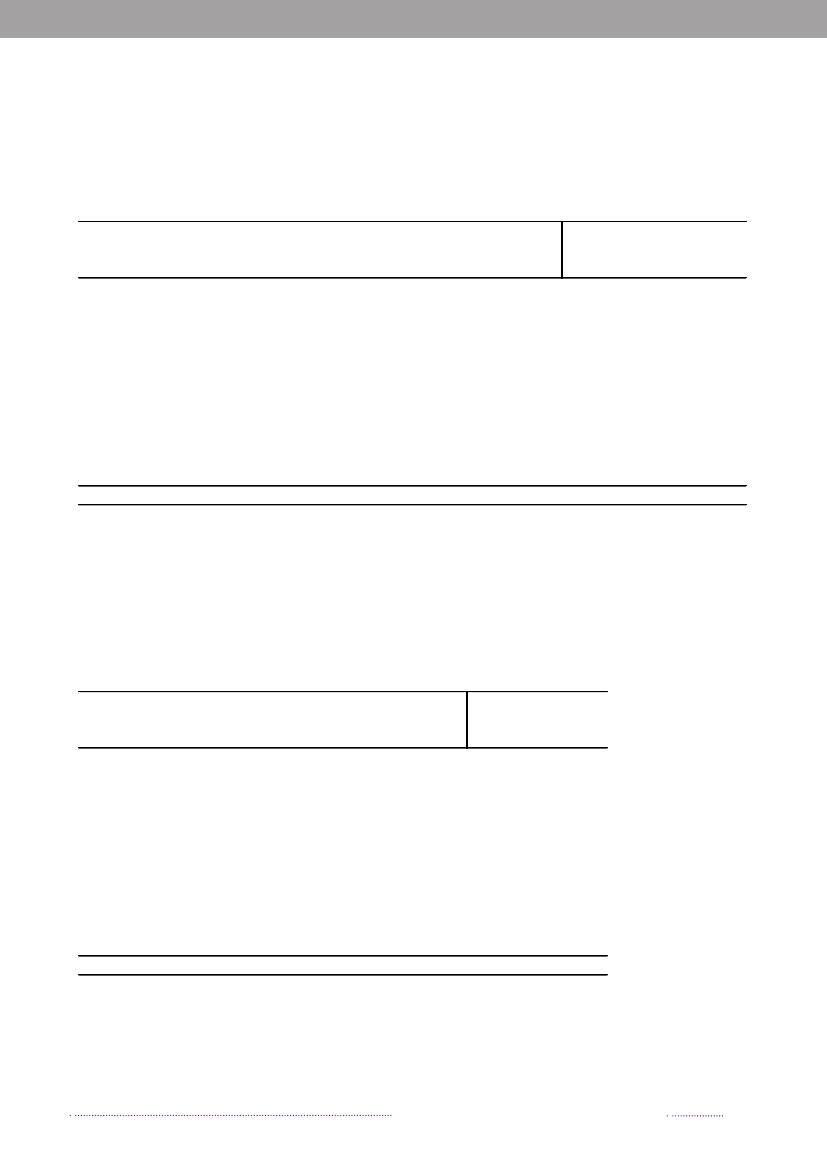
years. The proportion of cases that can be attributable tothe major food sources is presented in Figure 1.1.The incidence of human salmonellosis in 2008 was66.8 cases per 100,000 inhabitants (11.7 forS.Enteritidisand 36.6 forS.Typhimurium) (appendix B, Table A2).The estimated mean incidence per 100,000 inhabitantsattributed to the various food-animal sources was 28.9 forcases related to outbreaks, 15.6 for cases related to travel,5.8 for pork, 3.5 for imported chicken, 2.1 for table eggs,0.9 for broilers, 0.7 for ducks, 0.7 for imported pork, 0.5for beef, 0.2 for imported beef and 8.8 for cases related tounknown sources.In 2008, 1.572 human salmonellosis cases (43.0% ofall cases) were associated with outbreaks. The numberof outbreak-related cases that could not be linked to anysource constituted 39.6% (Figure 1.2). This represents asubstantial increase when compared to previous years, andis explained by the occurrence of one large long-lastingoutbreak caused byS.Typhimurium U292 and also severalsmaller outbreaks (appendix A, Table A1). See Chapter 2for further information on the outbreaks.
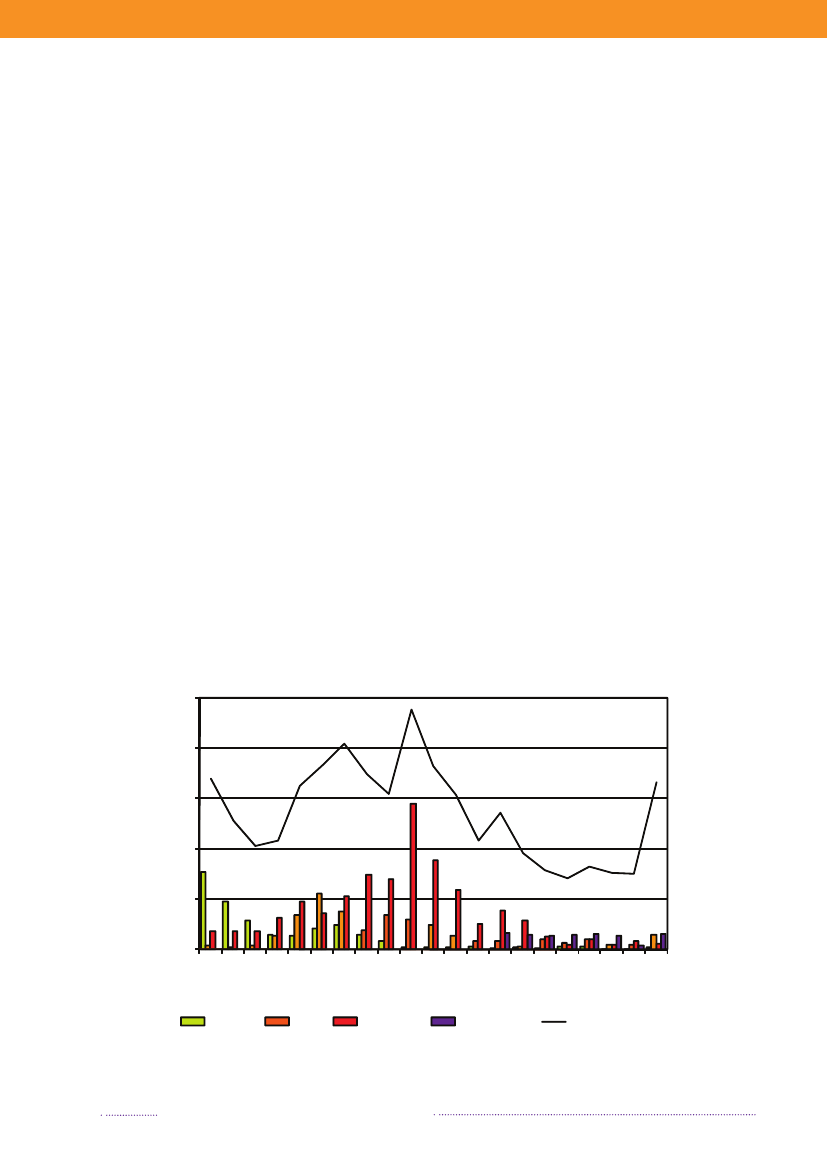
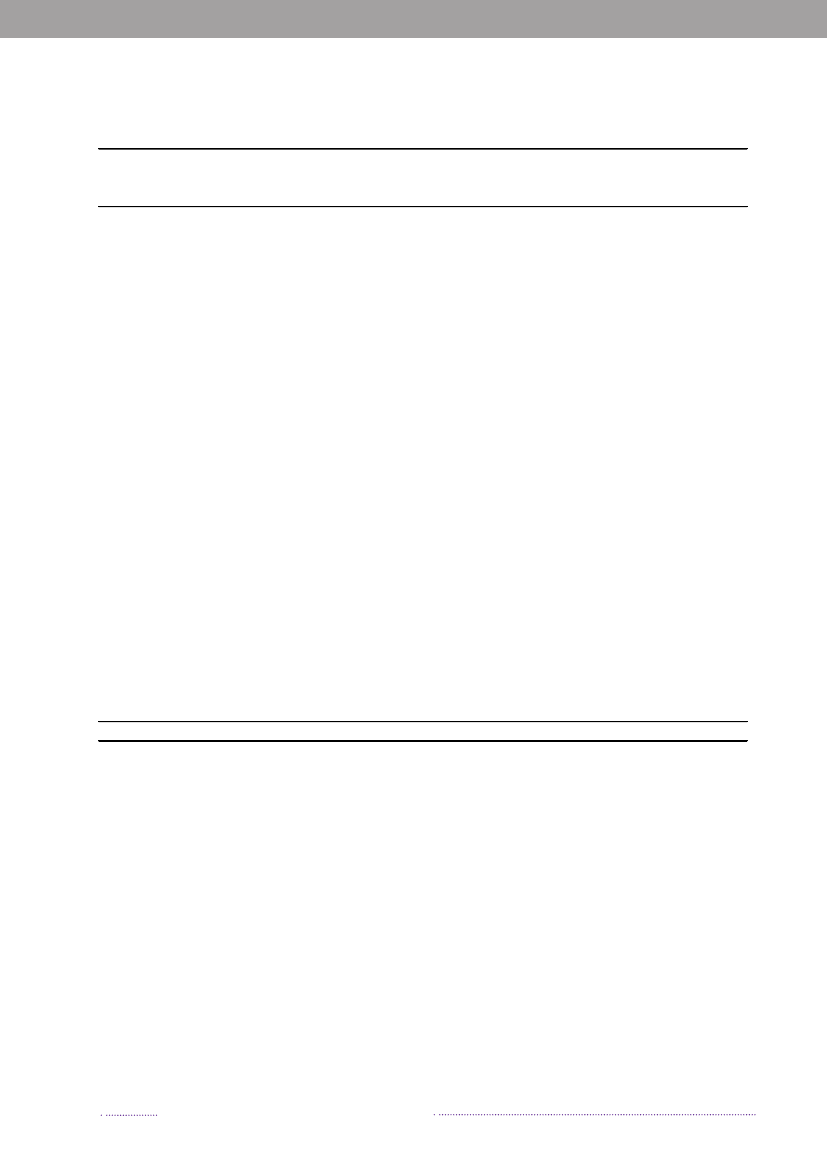
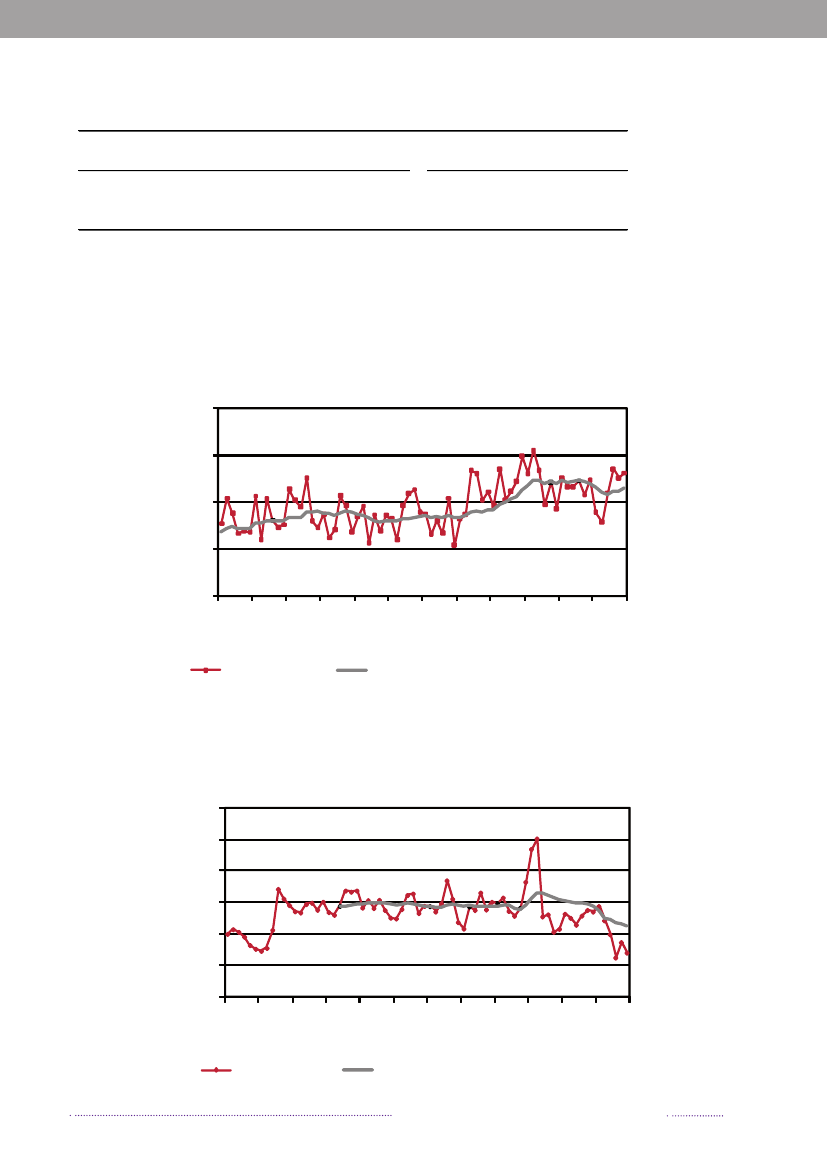
Figure 1.1. Total incidence of human salmonellosis and estimated human incidence due to broilers, pork,table eggs and imported foods in Denmark, 1988 to 2008100.080.0
Incidence per 100,000
60.040.020.00.088 89 90 91 92 93 94 95 96 97 98 99 00 01 02 03 04 05 06 07 08BroilersPorkTable eggsTotal importTotal cases
Source: Danish Zoonosis Centre, National Food Institute, Technical University of Denmark
6
Annual Report on Zoonoses in Denmark 2008
In total, 23.3% of all reported cases were estimated tobe acquired abroad; among the 2,084 reported sporadiccases (excluding outbreak related cases), it was estimatedthat 41.0% of the cases were acquired abroad. In 2008,Statens Serum Institut attempted to interview all patientswith diagnosedSalmonellainfections and no existing travelinformation. The patients were asked about the date of di-sease onset and whether they had travelled abroad withina seven-day period prior to disease onset. These data wascomplemented with information from general practitio-ners’ reports. Travel information was available from a totalof 86% of the reported cases. Among all reported cases ofsalmonellosis, 853 were estimated to be acquired abroad.Pork was estimated to be the most important source ofsalmonellosis in 2008 (8.8%), followed by imported chicken(5.2%) and table eggs (3.2%) (appendix A, Table A1). Theestimated number of cases attributed to the consumptionof pork increased three-fold compared to 2007. This in-crease is partly explained by the occurrence of an unusualnumber of pork-related outbreaks in 2008. Likewise, thecontribution of imported chicken to human salmonellosismarkedly increased in 2008, and the number of cases at-tributed to this source was three times higher than in 2007.The relative contribution of table eggs to salmonellosis inhumans decreased with 35.9% from 181 cases in 2007 to116 in 2008.
In 2008, 547Salmonellacases were attributed to dome-stic products, which is an increase compared to 2007 where312 of the cases were attributed to domestic products. Theincrease is mainly due to the increase in the number ofcases attributed to pork.In total, 329 of all cases could be attributed to importedfoods in 2008. The number of cases associated with im-ported foods almost tripled from 114 cases in 2007 and isat the same level as in 2006 (Appendix A, Table A1). Theincrease is mainly due to an increase in the number ofcases atributed to imported chicken and to a lesser extentimported turkey. Despite of the increase in total numberof cases due to imported foods, the relative proportionof cases attributed to imported foods decreased in 2007and 2008 to half the level compared to 2006. This is partlyexplained by the improved travel information obtained inthe last two years and, in 2008, by the many cases associatedwithSalmonellaoutbreaks. There is a considerable overlapbetweenSalmonellasubtypes isolated from patients thatacquired the infection abroad and from imported foods.The improved travel information allowed for the more ac-curate identification of international travel as the sourceof infection.The proportion of sporadic cases with unknown sourceof origin decreased from 23.4% in 2007 to 13.1% in 2008.This decrease is explained by the overall weight of out-
Figure 1.2. Estimated sources of 3,656 cases of human salmonellosis in Denmark, 2008(See also Appendix A, Table A1)
Source: Danish Zoonosis Centre, National Food Institute, Technical University of Denmark
Annual Report on Zoonoses in Denmark 2008
7
Trends and sources in human salmonellosis
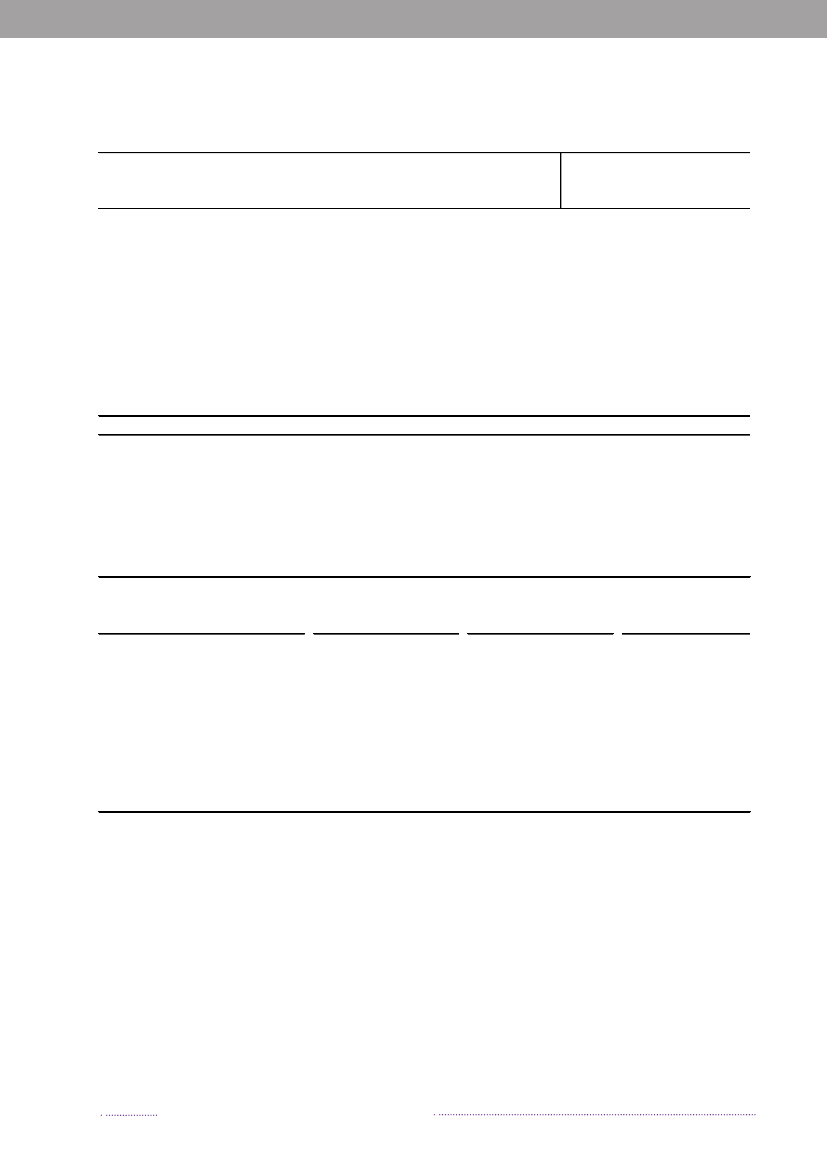
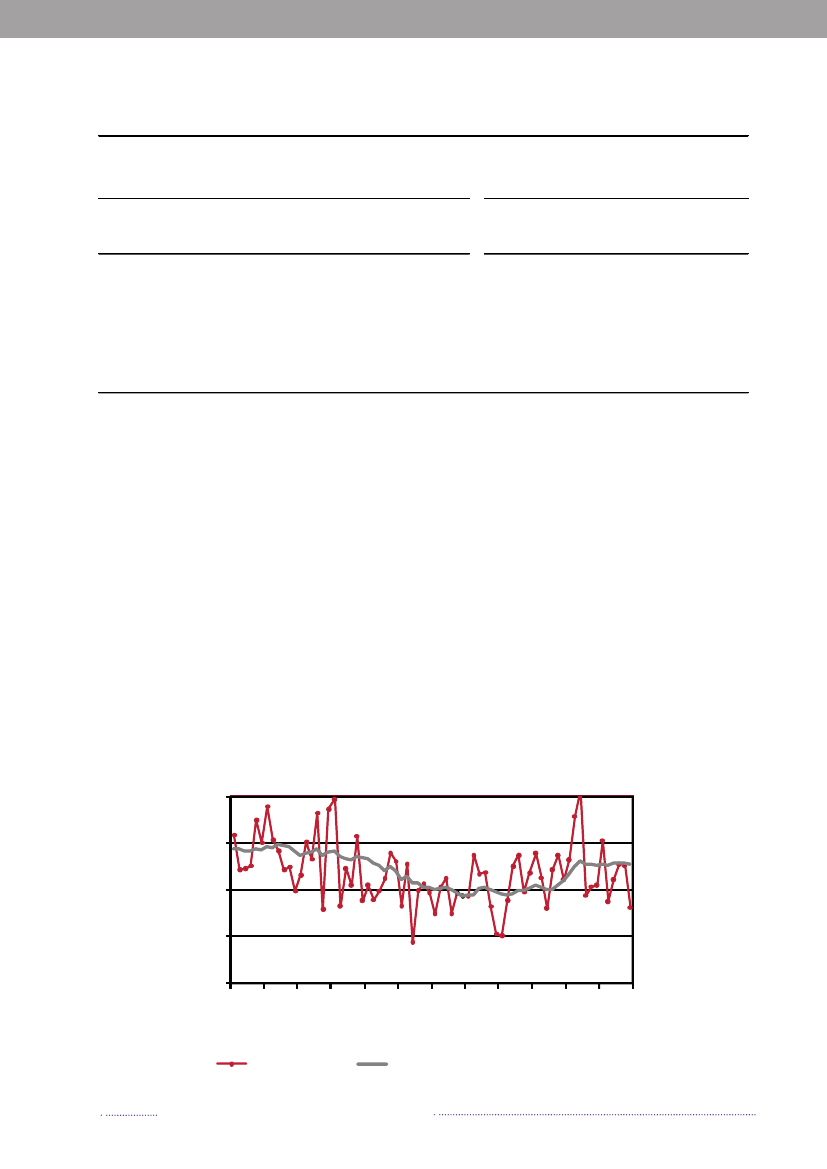
break-related cases in the total number of reported cases ofsalmonellosis. When analysing sporadic cases only, 23.0%ofSalmonellacases could not be attributed to any knownsource, similar to 2007. These cases may be caused by foodsnot included in the national surveillance (e.g. imported ordomestically produced fruits and vegetables), or by non-food sources of infection.Of the 638S.Enteritidis cases, 60.8% was estimated tobe related to international travel and only 8 cases (1.3%)were associated with outbreaks. Among the 2,002S.Typhi-murium cases, 74.0% was part of outbreaks and only 6.5%was estimated to be acquired abroad.The majority ofS.Typhimurium cases (75.0%) attribu-table to domestic products was caused by types susceptibleto all antimicrobials, whereas 21.0% was caused by typesresistant to one to three antimicrobial drugs, and 3.9% wascaused by types resistant to four or more antimicrobialdrugs (multi-resistant). In contrast, 38.8% of infectionsattributed to imported foods was caused by resistant typesand 24.4% by multi-resistant types. The same trend was
observed in cases acquired abroad, where the majorityof cases was caused by resistant and multi-resistant types(35.8% and 25.5%, respectively). Additionally, 11.9% oftravel-related cases was caused byS.Typhimurium isola-tes resistant to quinolones. This resistance profile was notfound in any of the Danish isolates.The number of human cases of salmonellosis reportedin 2008 reached a level not observed since the late 90’s.This increase was mainly due to the unusual high numberof outbreak-related cases, where most cases could not beattributed to any source. However, the number of cases at-tributable to food sources like Danish pork and importedpoultry products also increased substantially in 2008.References(1) Pires SM, Hald T (2009). Assessing the differences inpublic-health impact ofSalmonellasubtypes using a Ba-yesian microbial subtyping approach for source attribution.Foodborne Pathogens and Diseases, 7(2).
Figure 1.3. Sources of antimicrobial resistantS.Typhimurium infections in humans, 2004-2008200820072006200520042008200720062005200420082007200620052004050No. of cases100150Quinolone-resistant S. Tm.Susceptible S. Tm200
DK
Import
Travel
Multi-resistant S. TmResistant S. Tm
Source: Danish Zoonosis Centre, National Food Institute, Technical University of Denmark
8
Annual Report on Zoonoses in Denmark 2008
Trends and sources in human salmonellosis
Annual Report on Zoonoses in Denmark 2008
9
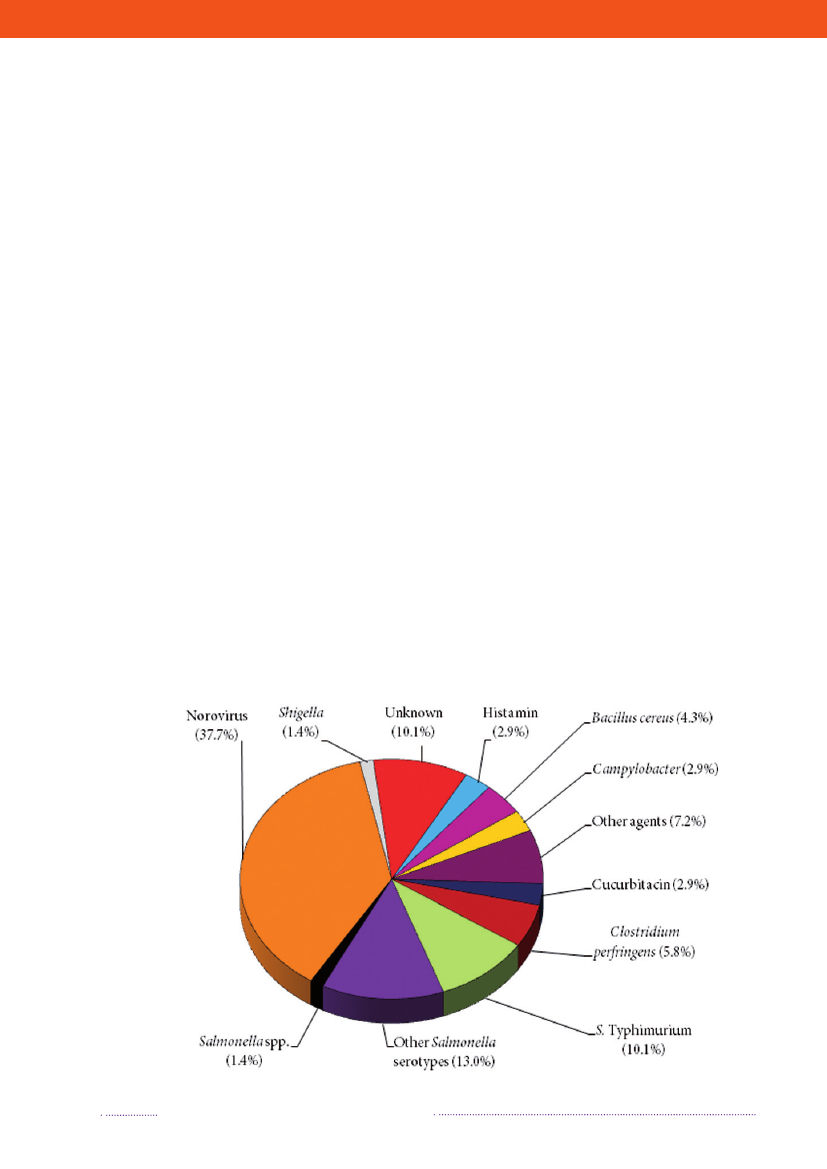
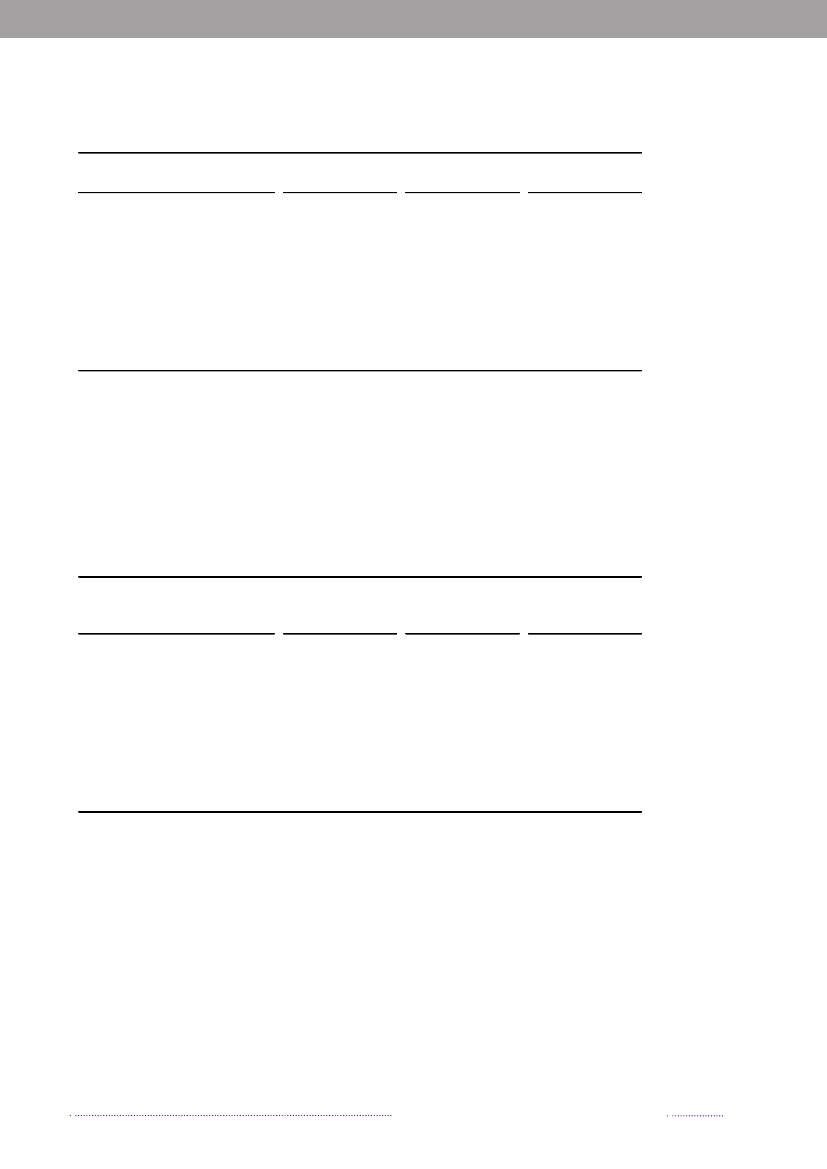
2. Outbreaks of special interestBy Steen Ethelberg ([email protected])In Denmark, foodborne outbreaks are investigated by anumber of different institutions, depending on the natureof the outbreak. Large, cross-regional foodborne outbreaksare typically investigated by Statens Serum Institut, the Na-tional Food Institute, Technical University of Denmark andthe Danish Veterinary and Food Administration, whereaslocal outbreaks are handled by the Regional Veterinaryand Food Control Authority in collaboration with themedical officer.Outbreaks are reported in the Food- and waterborneOutbreaks Database (FUD) and all verified outbreaks in2008 are presented in Appendix B, Table A4. Householdoutbreaks are not included in the table. The relative distri-bution of the outbreaks due to the different pathogens ispresented in Figure 2.1. The reporting and outbreak inve-stigation systems are described in further detail in Chapter6.2. Some of the more notable outbreaks are outlined below.2008 was an unusual year because of a very large out-break ofSalmonellaTyphimurium. A total of 1,224 casesofS.Typhimurium U292 belonging to the same MLVAcluster (FUD no. 788) were registered in what was thelargest knownSalmonellaoutbreak in Denmark to date(1-4). The outbreak was detected April 1stand during thesummer 30 to 60 new cases appeared every week, graduallydecreasing over the autumn and winter. This outbreak hasbeen the subject of a very large and intensive investigation,among others including measures such as:• A large number of trawling questionnaires• Case-control and cohort analyses• Investigations of a number of slaughter houses andfood production facilities• Comparative molecular subtyping of relevant isolatesfrom many different sources• Structured microbiological analyses of food samplesfrom patients homes• Investigation of shopping records obtained fromsupermarket computers• Epi and trace-back analyses of embedded outbreakswhere several persons have been ill when participa-ting in the same event• Risk ranking of slaughterhouses, shops food items andanimal herds.
Figure 2.1. Aetiology of foodborne disease outbreaks reported with a causative agent in the Food- andwaterborne Outbreak Database (FUD), 2008. Percentage of total outbreaks indicated in brackets
Source: Statens Serum Institut
10
Annual Report on Zoonoses in Denmark 2008
As of November 2009, the source of this outbreak isstill not known. Only very few cases with the outbreakstrain have been detected outside of Denmark and themain hypothesis remains that the outbreak is caused by aseries of different foods and originates from a pig reservoir.Two other relatively large outbreaks caused byS.Typhi-murium strains belonging to the otherwise rare phagetypes DT135 (FUD no. 854) and DT3 (FUD no. 853) alsooccurred and also remain unsolved (2). Both outbreaksshow similarities with the U292 outbreak and may sharethe same underlying cause.Several other outbreaks withS.Typhimurium took placeas well. One outbreak with phage type DT120 comprised53 cases and occurred during June and July. This outbreakwas solved as a spin-off of the U292 outbreak investigationwhen smoked ham sampled from the home of a case (thefood samples were collected prior to MLVA typing) wasfound positive for the DT120 outbreak strain.Two outbreaks with phage type U288 occurred; theywere unrelated and belonged to different MLVA types.The first of these outbreaks was localised to central Jutlandand comprised 37 cases (among which were two Norwe-gian tourists) in the spring of 2008 (FUD no. 793); it wastraced back to a small group of shawarma restaurants. Thesecond U288 outbreak occurred in the autumn and winterof 2008 and comprised 39 registered cases (FUD no. 855)most of whom lived on Zealand; four of the patients died.This outbreak was caused by pork in different forms; theoutbreak strain was isolated from pork meat from differentfood producers that were supplied from the same slaughter-house where the outbreak strain was also isolated. Part ofthe contaminated pork meat was sold to Sweden and gaverise to illness in both Sweden and Norway (5).Finally, a comparable outbreak with phage type U312took place in the winter 2008/09 (FUD no. 863). Intotal, it comprised 42 cases (of which some occur-red in 2009) and this outbreak was also causedby different forms of pork meat that origi-nated from a specific slaughterhouse (6).Norovirus is not a zoonosis, but itshould be mentioned that, as in previous years,norovirus was the most frequent disease agentin the registered outbreaks. Of the 69 foodborneoutbreaks in 2008, norovirus accounted for 26 with anaverage of 30 patients. These outbreaks were generally aresult of contamination events associated with workplacelunch buffets, restaurants and private parties. Several ofthese outbreaks followed gastrointestinal symptoms inpersons preparing the food.
References(1) Ethelberg S, Wingstrand A, Jensen T, Sørensen G,Müller L, Lisby M, Nielsen EM, Mølbak K (2008). Largeoutbreaks ofSalmonellaTyphimurium infection in Den-mark in 2008. Euro Surveill; 13(44).(2) Ethelberg S, Wingstrand A, Jensen T, Sørensen G,Müller L, Nielsen EM, Mølbak K (2008). Large ongoingoutbreak of infection withSalmonellaTyphimurium U292in Denmark, February-July 2008. Euro Surveill 13(28).(3) Statens Serum Institut (2008): Epi-news, week 27-33.(4) Statens Serum Institut (2008): Epi-news, week 49.(5) Bruun T, Sørensen G, Forshell LP, Jensen T, NygardK, Kapperud G, Lindstedt BA, Berglund T, Wingstrand A,Petersen RF, Müller L, Kjelsø C, Ivarsson S, Hjertqvist M,Löfdahl S, Ethelberg S (2009). An outbreak ofSalmonellaTyphimurium infections in Denmark, Norway and Swe-den, 2008. Euro Surveill; 14(10).(6) Statens Serum Institut (2009): Epi-news, week 9.
Annual Report on Zoonoses in Denmark 2008
11
Outbreaks of special interest
3. Typing methods in the surveil-lance of foodborne pathogensBy Eva Møller Nielsen ([email protected]), Gitte Sø-rensen, Dorte Lau Baggesen and Karl PedersenCharacterisation – or typing – of bacterial isolatesis an important tool for surveillance, source finding,outbreak detection, outbreak investigations, and sourceattribution. The methods used range from conventio-nal phenotypic methods, such as serotyping and phagetyping, to high-discriminatory molecular methods.The development of new, faster and cheaper molecularmethods in recent years has made it possible to usethese methods as an integrated part of surveillance offoodborne infections in human patients as well as in thefood chain. Here, we briefly describe how the methodsare used in the surveillance of foodborne pathogens inDenmark and how this has developed in recent years.still taking place at all production stages, i.e. breeding andparent flocks, table egg layers and broilers. Every flock istested forSalmonellaby culture (breeding and parent flocks,layers and broilers) and serology (layers). See appendix D,tables A32-A34 for detailed information on surveillanceprogrammes. All poultry isolates positive forSalmonellamust be submitted to the National Veterinary Institute,Technical University of Denmark for serotyping. IsolatesofS.Typhimurium andS.Enteritidis are then forwardedto the National Food Institute for phage typing.Only very few flocks of duck or turkey are slaughte-red in Denmark, as most are exported as live animals.Isolates collected before slaughter are analysed at privatelaboratories and positive isolates are sent to the NationalVeterinary Institute.In pigs, there is a serologicalSalmonellasurveillanceprogramme in slaughter pig herds based on meat juicesamples collected at the slaughterhouse, and in breeder andmultiplier herds based on monthly blood samples from allherds. See appendix D, Table A37 for detailed informationon the surveillance programmes. A highSalmonellaindexresults in compulsory bacteriological examination of faecalsamples from the pens. Isolates from these samples aresent for typing to the National Food Institute. A highSal-monellaindex in the slaughter pig herds will also result incompulsory collection of pen faecal samples in sow herdsthat have delivered pigs to the slaughter pig herd. This isdone to clarify distribution and type of infection in theherd and also to clarify possible transmission from sowherds to slaughterpig herds.In cattle,Salmonellasurveillance is active in the primaryproduction on the basis of serology specifically directedagainstS.Dublin. See appendix D, Table A36 for detailedinformation on the surveillance programme. In the dairysector, serology is based on tank milk samples, and in beefcattle, serology is based on blood samples.Clinical outbreaks of salmonellosis occasionally occur inpig and cattle herds, most often caused byS.Dublin (cattle)orS.Typhimurium, and isolates from such outbreaks aresubmitted to the National Food Institute for serotyping,phage typing (S. Typhimurium andS.Enteritidis) andantimicrobial susceptibility testing.In food, there is surveillance forSalmonellain meat, inparticular on pig and cattle carcasses at the slaughterhouses,however, other food products, both domestic and importedfoods, are also wxamined. Isolates ofSalmonellamust be
3.1 Surveillance of human infectionsFor some of the gastrointestinal infections, the bacterialisolates undergo a routine characterisation in the primarylaboratory - typically at a regional clinical microbiologicallaboratory - where the pathogen is cultured from stoolsamples. For example, the two most prevalentSalmonellaserotypes (S. Enteritidis andS.Typhimurium) are usuallydetermined at this stage. For others, no characterisation ismade beyond species or even genus level, e.g. thermophi-licCampylobacterare designatedC. coli/jejuniwithoutspecies determination or any further typing. All isolatesofSalmonella,verocytotoxin-producingE. coli(VTEC),andListeriaare sent to Statens Serum Institut for furthercharacterisation (Table 3.1). Isolates ofS.TyphimuriumandS.Enteritidis are sent to the National Food Institute,Techical University of Denmark for phage typing.Cam-pylobacterisolates from three selected geographic areas,covering approx. 25% of the country, are submitted toStatens Serum Institut.
3.2 Surveillance of food and animalisolatesSalmonellasurveillance programmes are active on bothproduction animals and food, although with different in-tensity. The most thorough surveillance is carried out inthe poultry sector, where the Danish control programmefor surveillance and eradication ofSalmonellawas launch-ed in 1996. The programme has been modified severaltimes over the years and comprehensive surveillance is
12
Annual Report on Zoonoses in Denmark 2008
Outbreaks of special interest
Table 3.1. Methods used in the surveillance of foodborne pathogens in DenmarkIsolatesPathogenMethodsHumanFoodAnimalSalmonella entericaSerotypeAllAllAllPhage typeAntimicrobialresistanceMLVAPFGECampylobacter coli/jejuniAntimicrobialresistancePFGEMLSTVTECSerotypeVirulence profilePFGEListeriaSerogroupPFGEYersinia enterocoliticaO-groupAllAllIsolates from 1 districtNoneNoneNoneNoneNoneNoneAllAllAllAllAllNoneAllAllNoneS.Typhimurium andS.EnteritidisS.Typhimurium, 50% ofS.Enteritidis, approx.90% of other serotypesS.TyphimuriumOutbreak investigationsS.Typhimurium andS.EnteritidisS.Typhimurium andoccasionally otherserotypesS.Typhimurium(outbreak investigations)Outbreak investigationsS.Typhimurium andS.EnteritidisS.Typhimurium andoccasionally otherserotypesS.Typhimurium(outbreak investigations)Outbreak investigationsOnly for DANMAPsurveillance purposesNoneNone
Isolates from 3 districtsOnly for DANMAPfor DANMAP surveillance surveillance purposesOutbreak investigationOutbreaks investigaions,researchNoneNone
Source: Statens Serum Institut, Danish Zoonosis Laboratory and Danish Zoonosis Centre, National Food Institute, TechnicalUniversity of Denmark
submitted to the National Food Institute for typing.Salmonellaisolates submitted to or cultured by theNational Food Institute are serotyped on a routine basis.In the case of S. Typhimurium or S. Enteritidis findings,isolates are phage typed, and S. Typhimurium isolatesare also subjected to antimicrobial susceptibility testing.Further molecular typing is only carried out as a part ofoutbreak investigations and is coordinated with StatensSerum Institut.In addition toSalmonella,there is surveillance ofCam-pylobacterin broiler flocks at slaughter and on randomsamples of domestic and imported poultry meat. Samplesare analysed at authorised private laboratories or by theNational Veterinary Institute.There is a very limited surveillance of VTEC on samplescollected from fresh meat at cattle slaughterhouses, appro-ximately 200 samples per year. The samples are analysed atthe National Food Institute. Isolates – if any – are subjectedto serotyping and toxin typing. No other routine typing
is performed on bacterial pathogens of food or veterinaryorigin (Table 3.1).
3.3 Outbreak detection and investiga-tionMolecular typing of allS.Typhimurium, VTEC andListeriaisolates from human infections is carried out asroutine investigations and used for outbreak detection.For the majority of investigated outbreaks with thesepathogens, the detection of a cluster of isolates by the useof molecular methods is the first indication that triggersfurther outbreak investigations. Since mid-2004, bothphage typing (1) and the high-discriminatory typingmethod MLVA (Multiple Locus VNTR Analysis) (2) havebeen used as routine typing of allS.Typhimurium isola-tes from humans. Prior to 2004,S.Typhimurium isolateswere routinely phage typed and from 2003 to 2006 PFGE(Pulsed-Field Gel Electrophoresis) typed as well (3). Howe-ver, the discrimination obtained by MLVA is significantlyAnnual Report on Zoonoses in Denmark 2008
13
Typing methods
higher than by PFGE and the introduction of the MLVAmethod has lead to an increase in the number of detectedand solvedS.Typhimurium outbreaks in recent years (4).The first indication of an outbreak caused by morerare serotypes ofSalmonellaor byYersinia, ShigellaandCampylobacteretc. is an increase in the total number ofinfections with one of the pathogens. In order to confirmthe outbreak and define the outbreak related cases, mole-cular typing will typically be performed on isolates fromhuman cases diagnosed during a specific time period(Table 3.1). See Chapter 6.1 for more detailed informationon the reporting system.Although all human infections caused by possiblefoodborne pathogens are centrally registered, the isolatesof other organisms thanSalmonellaand VTEC are notalways available for typing as they might be discarded afterspecies confirmation at the regional laboratories.When a cluster of human infections with a specific pat-hogen is detected, the National Food Institute will searchtheir database for relevant animal or food isolates that maycome from a possible source. If the pathogen has beenregistered in any animal or food sample, a hypothesis ofthe typical reservoir of the specific pathogen may becomeclear and isolates will undergo further typing to confirma possible match to the human type. ForSalmonellaout-breaks, this search is typically based on the phage typeand resistance profile. Isolates matching at this level areMLVA typed and/or PFGE typed and compared to theoutbreak type.
If an outbreak is suspected to have an internationalaspect, international alerts or inquiries are sent out viaPulseNet and the European Centre for Disease Preventionand Control. Further, in relation to identification of a pos-sible animal reservoir or food source, inquiries are sentout in the network of Community Reference Laboratories(CRLs) and National Reference Laboratories (NRLs). Inorder to be able to extrapolate results between countries, itis important that the molecular typing data are comparablebetween laboratories and countries.References(1) Anderson ES, Ward LR, Saxe MJ, de Sa JD. (1977).Bacteriophage-typing designations ofSalmonellaTyphi-murium. J. Hyg. (Lond); 78:297-300.(2) Lindstedt BA, Vardund T, Aas L, Kapperud G.(2004). Multiple-locus variable-number tandem-repeatsanalysis ofSalmonellaenterica subsp. enterica serovarTyphimurium using PCR multiplexing and multicolorcapillary electrophoresis. J Microbiol Methods; 59:163-172.(3) Ribot EM, Fair MA, Gautom R, Cameron DN, Hun-ter SB, Swaminathan B, Barrett TJ. (2006). Standardizationof pulsed-field gel electrophoresis protocols for the subty-ping ofEscherichia coliO157:H7,Salmonella,andShigellafor PulseNet. Foodborne Pathog Dis; 3:59-67.(4) Torpdahl M, Sørensen G, Lindstedt BA, NielsenEM. (2007). Tandem repeat analysis for surveillance ofhumanSalmonellaTyphimurium infections. Emerg InfectDis; 13:388-395.(5) Swaminathan B, Gerner-Smidt P, Ng LK, LukinmaaS, Kam KM, Rolando S, Gutierrez EP, Binsztein N. (2006).Building PulseNet International: an interconnected systemof laboratory networks to facilitate timely public healthrecognition and response to foodborne disease outbreaksand emerging foodborne diseases. Foodborne Pathog Dis;3:36-50.(6) Larsson JT, Torpdahl M, Petersen RF, Sørensen G,Lindstedt BA, Nielsen EM. (2009). Development of a newnomenclature for Salmonella Typhimurium multilocusvariable number of tandem repeats analysis (MLVA). EuroSurveill;14(15).
3.4 National and internationalcooperationThe typing methods used for the central surveillanceand outbreak investigations taking place at Statens SerumInstitut, the National Food Institute and the NationalVeterinary Institute are fully standardised and compara-ble between the laboratories. ForSalmonella,the shareddatabase with molecular typing data is an important toolin the surveillance.The phenotypic and molecular typing methods areinternationally recognised methods and most of themare standard methods with international external qualityassurance programmes, e.g. serotyping ofSalmonellaandVTEC, phage typing ofSalmonella,antimicrobial resistanceprofiling ofSalmonellaandCampylobacter.The moleculartyping methods are also international methods, however,not standardised to the same level as the other methods.For PFGE typing, the PulseNet US protocols are used (5).The method for MLVA typing ofS.Typhimurium is usedin several other European countries. This method needsfurther standardisation for the results to be fully compa-rable between laboratories and this work is ongoing (6).The Danish laboratories are actively involved in the inter-national development and cooperation on typing methods.
14
Annual Report on Zoonoses in Denmark 2008
Typing methods
Annual Report on Zoonoses in Denmark 2008
15
Typing methods
4. VTEC - status and human riskfactorsBy Lone Jannok Porsbo ([email protected]),Jeppe Boel and Flemming ScheutzEscherichia coliare part of the normal micro flora of thegastrointestinal tract of mammals and birds, but certaingroups ofE. colihave been associated with gastrointestinaldiseases in both humans and animals. Verocytotoxin-producingE. coli(VTEC) is a group ofE. colithat is cha-racterized by the ability to produce verocytotoxins (VT).Human pathogenic VTEC usually harbour additionalvirulence factors that are important for the developmentof disease and a large number of serogroups ofE. colihave been recognized as verocytotoxin producers. Thetwo main types of VTs are encoded by the genesvtx1andvtx2,withvtx2being the most virulent type. Anotherimportant virulence gene found in VTEC is theeaegene,coding for the production of intimin. Most human VTECinfections are associated with a limited number of O:Hserotypes. Of these, VT2-producing E. coli O157:H7 andO157:H (VTEC O157) are the most frequently reportedtypes associated with human disease (1).The majority of reported human VTEC infectionsare sporadic cases and are associated with watery orbloody diarrhoea (haemorrhagic colitis), which is oftenaccompanied by abdominal cramps, usually withoutfever. The registered cases are mainly children less than5 years of age. VTEC infections can result in haemolyticuraemic syndrome (HUS), which is characterized byacute renal failure, anaemia and lowered platelet counts.HUS develops in up to 10% of patients infected withVTEC O157 and is the leading cause of acute renal failurein young children. VTEC infection is lethal in 2 – 7 % ofthe cases (2). In Denmark, VTEC infections, includingHUS, has been notifiable since 2000. From 2000 – 2008, 38cases developed HUS corresponding to 3.1% of all patientsinfected with VTEC.VTEC (including VTEC O157) has been isolated frommany different animal species. The gastrointestinal tract ofhealthy ruminants is the primary reservoir for VTEC, andfoods of bovine origin are frequently reported as a sourceof human VTEC infections. The infectious dose is low andhuman infections can be acquired through the consump-tion of contaminated food or water, by direct transmissionfrom person to person or from direct contact with infectedanimals. The importance of many of the VTEC types thatcan be isolated from animals and foodstuffs for infectionsin humans is not yet clear.
4.1 Human cases in 2008In 2008, there were 161 reported VTEC cases; an in-cidence of 2.9 per 100,000 inhabitants. VTEC cultureswere obtained from 158 cases (some were diagnosed byPCR only, some were notifications from one of the districthospitals), of which 9% were caused by O157. The totaldistribution of VTEC O-groups, resulting in five or moreepisodes, is presented in appendix B, Table A3.The number of registered infections was unchangedfrom 2007 to 2008. This follows several years of increasein the number of infections from the beginning of surveil-lance in 1997 to 2007 (appendix B, Table A2). The increase
16
Annual Report on Zoonoses in Denmark 2008
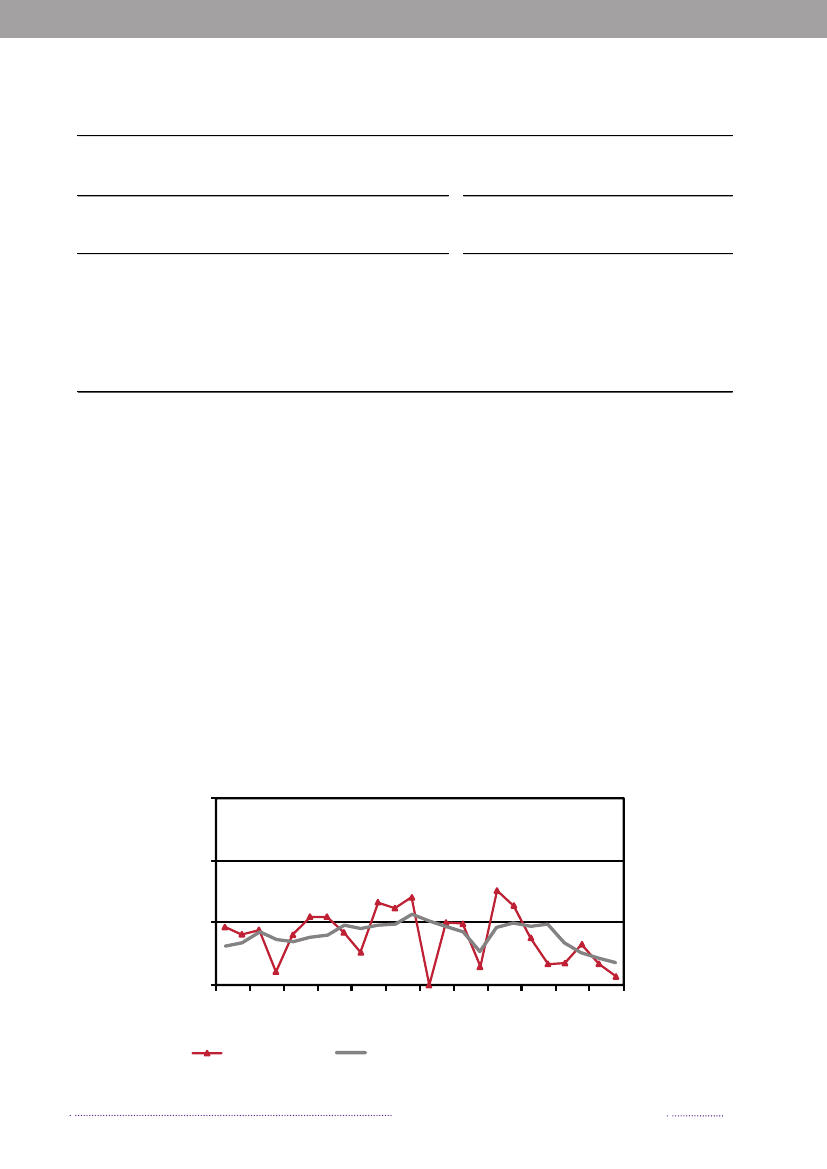
Figure 4.1. VTEC O157-prevalence in cattle, 1999-2008
1210
Prevalence (%)
8642019992000200120022003 2004Year2005200620072008
Source: National Food institute, Technical University of Denmark
is primarily assumed to reflect improved diagnostics andincreased awareness. However, Denmark does not have acentrally coordinated standard testing method for VTECand the incidence through the past 10 years has been 3 to10 times higher in laboratory uptake areas using a diag-nostic approach involving molecular detection methods(appendix B, Figure A5). A rough estimate indicates thata little more than 50% of the Danish population was co-vered by molecular detection methods in 2008. In 2008,the age group specific incidence in areas using molecularmethods was estimated to be 19.0 per 100,000 inhabitantsin children less than 5 years and 3.9 per 100,000 inhabitantsin the group with cases aged 5 years or more comparedto 3.7 and 0.7, respectively, in areas using other methods.There were four cases of VTEC related HUS in 2008.Unusually, all four cases were associated with non-O157serotypes; O145:H- (2 boys aged 3 and 5 years), O111:H-(1 girl aged 2 years) and O121:H19 (1 girl aged 1 year).All cases were positive for theeaegene andvtx2.TheO111:H- strain was also positive forvtx1.In 2008, allVTEC isolates were real-time sub-typed using PFGE atStatens Serum Institut.Foodborne outbreaks caused by VTEC are rare inDenmark and in 2008, no general outbreaks occurred. In2007, organic salami made from beef contaminated withVTEC O26:H11 caused a six week long outbreak (seealso Annual Report 2007). During this period 20 cases,mainly small children (1 – 3 years of age) were laboratoryconfirmed. The symptoms were in general mild and onecase of haemorrhagic colitis occurred. The epi-type wasdetected from the batch of imported frozen beef used toproduce the salami. For the first time in Denmark, datafrom credit cards were used together with a case-controlstudy in the epidemiological investigation (3). In 2004, anoutbreak with VTEC O157 was epidemiological connected
to drinking pasteurized milk from a certain dairy (see alsoAnnual Report 2004) (4).
4.2 VTEC in cattleThe National Food Institute, Technical University ofDenmark has monitored the occurrence of VTEC O157in cattle since June 1997 through yearly examinations ofapproximately 200 fecal samples from slaughter calves. Thesamples are collected at the slaughterhouses as part of theDANMAP programme. The samples (25 g) are analysedby overnight enrichment followed by immunomagneticseparation and seeding on to sorbitol MacConkey agarsupplemented with cefixime and potassium tellurite.Isolates ofE. coliO157 are analysed for genes encodingverocytotoxins by PCR analysis.In 2008, VTEC O157 was detected in 7.2% (16/222) ofthe analysed samples. This prevalence is in line with thefindings in previous years, where the observed prevalencehas ranged from 2.8% to 10.3% with an average prevalenceof 5.7% (Figure 4.1).
4.3 VTEC in minced beef – 2008 investi-gationIn 2008, the Danish Veterinary and Food Administra-tion conducted a VTEC survey of 263 samples of mincedmeat including 32 veal and 231 beef samples. The sampleswere collected at the retail level and the analyses wereperformed by the Regional Food Laboratory (furtherdescribed in Chapter 6.4). The samples were enriched byovernight incubation. DNA was purified from the enrichedculture and analysed for genes encoding verocytotoxin(vtx1 andvtx2)and anE. coliserogroup O157 specificgene by real-time PCR analysis. The samples were regar-ded as real-time VTEC/O157 positive if they exhibited aCt value of <30.Annual Report on Zoonoses in Denmark 2008
17
VTEC
Attempts were made to isolate VTEC from 21 samplesthat exhibited Ct values <25 in thevtxreal-time PCR as-say. The primary BPW enrichment broth was seeded ontryptone bile x-glucuronide (TBX) agar. Approximately 10β-glucuronidase positive colonies per sample were exami-ned for VT encoding genes by real-time PCR.Enrichment broths from samples that were O157 real-time PCR positive were further examined as specifiedin the ISO 16654:2001 standard. Isolates ofE. coliO157were analyzed for verocytotoxin-encoding genes byvtxreal-time PCR.Thirty-three of the 263 samples (12.5%) were positive bythevtxreal-time PCR assays; 17 samples werevtx1positiveand 28 samples werevtx2positive. VTEC was isolated fromfour samples. Four samples were O157 real-time PCR po-sitive and three of these samples were also positive in thevtxreal-time PCR assay. VTEC O157 were isolated fromtwo (0.8%) of the investigated samples.
Clinical symptoms, complications and hospitalizationswere described for 73 cases interviewed in connection withthe study. In total, 26.0% of cases was hospitalized in 3.5days on average; 8.2% of cases developed HUS and 35.6%haemorrhagic colitis. The most frequent symptom wasdiarrhoea (98.6%) and abdominal pain (64.4%). VTECO157 was the most frequent serotype (26%); all O157samples werevtx2andeaepositive;vtx1was only seenin connection withvtx2.VTEC O157 was responsible for66.7% of the HUS incidence and 57.9% of the incidencewith haemorrhagic colitis. The most common serotypesbesides O157 were O103, O26 and O145.
4.4 Risk factors for sporadic VTEC in-fections in DenmarkIn 2003–2005, Statens Serum Institute and the NationalFood Institute conducted a case-control study, which wasthe first systematic investigation of risk factors for sporadicVTEC infections in Denmark. The study was a matchedcase-control study with cases and controls matched onsex, age and municipality. Information on medical history,travel history, food exposures and contact to animals werecollected through telephone interviews.Cases and controls were excluded:• If they had been travelling outside Denmark intwo weeks prior to the first symptoms of infection• If they had been part of an outbreak• If the interview resulted in insufficient informationto fulfill the criteria to be included in the study.Before excluding travel associated cases, matched univa-riate analysis was conducted and foreign travel was foundto be a significant risk factor for VTEC-infection, withEgypt and Turkey as the most frequently visited countries.The final analysis contained 57 cases and 118 matchedcontrols. Half of the cases were females (52.6%) and themedian age was 4 years (age range 0–77 years). The pre-liminary multivariate analysis pointed out two exposuressignificantly associated with VTEC infection: Eatingstrawberries or having experienced irregularities with thedomestic water supply, i.e. bad smell or soil in the water.The two risk factors were independent of season, andthe domestic water supply was in all cases stated to bepublic water supply. The largest subgroup of cases was 27children 0 – 3 years of age. Matched multivariate analysiswas conducted separately for this group of cases and tworisk factors were found: Drinking unpasteurized milk andhaving had contact to ruminants.
References(1) Duffy G (2006). Emerging pathogenic E. coli. In:Motarjemi Y, Adams M, red. Emerging Foodborne Patho-gens. Woodhead Publishing Ltd. 10: 253-281.(2) Nelson S, Clarke RC, Woods LM (1998). Verocyto-toxin-producingEscherichia coli(VTEC) infections. In:Palmer SR, Lord Soulsby, Simpson DIH red. Zoonoses.Oxford Medical Publications, p. 89-104.(3) Ethelberg S, Smith B, Torpdahl M, Lisby M, Boel J etal (2009). Outbreak of Non-O157 Shiga Toxin-ProducingEscherichia coliInfection from consumtion of Beef Sausage.CID 48(15 April), Brief Report.(4) Jensen C, Ethelberg S, Gervelmeyer A, Nielsen EM,Olsen KEP, Mølbak K, and the outbreak investigation team(2006). First general outbreak of Verocytotoxin-producingEscherichia coliO157 in Denmark. Euro Surveill 11(2).
18
Annual Report on Zoonoses in Denmark 2008
5. EU related topics5.1Trichinellaspecial statusIn July 2007, the European Commission and the otherMember States assigned Denmark status as a region wherethe risk ofTrichinellain domestic swine is officially recog-nised as negligible (EU Regulation (EC) No 2075/2005).As a result of this status the future monitoring pro-gramme forTrichinellacan be risk based. Slaughter pigsreared under controlled housing conditions in integratedproduction systems do not have to be tested forTrichinellaany more. All other categories of pigs and other species(domestic or game) which can become infected withTri-chinellawill be examined in accordance with the methodslaid down in Regulation (EC) No 2075/2005. Further,pork exported to third market countries will be tested forTrichinellaunless the importing country accepts the newmonitoring programme.In order to fulfil the requirements set out by the Regu-lation, a monitoring programme forTrichinellain wildlifemust be in place. This programme was initiated in 2008. Intotal, 300 foxes and 50 other carnivores will be examinedannually. In 2008,Trichinella pseudospiraliswas found intwo wild mink on the island of Bornholm. Due to that, alarge number of mink and crows were examined on Born-holm. There were no other findings. A report on results ofthe DanishTrichinellatesting and monitoring for 2008 hasbeen forwarded to the Commission.EU harmonised surveillance programmes2008 was the first year Member States was obliged toreport the prevalence in laying hen flocks based on a har-monised surveillance programme according to Regulation(EC) 2160/2003. Due to the large variation between Mem-ber States in prevalence of laying hen flocks reported in thebaseline study finalised in 2005 (See Annual Report 2006for overview of Danish results), the Commission decidedto set a Member State reduction target depending on theprevalence in the preceding year until the prevalence fallsbelow 2.0% (Regulation (EC) No 1168/2006). This targethas to be reached by December 31st2010. Denmark hashad intesiveSalmonellasurveillance programmes for manyyears and the target of 2.0% has already been reached.Survey on prion protein genotypes in sheepDenmark has a population of approximately 200,000sheep and lambs. In the sheep population, some animalshave a genotype resistant to classical scrapie. Although lessconclusive, evidence also suggests that the same genotypeis resistant to BSE. The pathogenic prion load in theseresistant sheep is much lower than in non-resistant sheep.Therefore, the resistant sheep will pose a much lower publichealth risk, compared to that of non-resistant sheep. In2008, like previous years, a study was conducted to deter-mine the prion protein genotypes from a sample of ovineanimals according to Regulation (EC) No 999/2001 (asamended). The study included 100 randomly selected ani-mals. Results showed that 29.4% of sheep had the resistantprion protein genotype ARR/ARR which is an increasecompared to last year where 16.0% of the sheep had theresistant prion protein genotype (Appendix C, Table A26).
5.2 Control ofSalmonellain animalpopulations– EU regulations and studiesEU Baseline studiesBased on the Zoonosis Directive 2009/99/EC and Regu-lation (EC) 2160/2003 the Commission has initiated EU-studies – the Baseline Studies - of theSalmonellaprevalencein laying hens, broilers, broiler carcasses, breeding pigs,slaughter pigs and turkeys, of theCampylobacterpreva-lence in broilers and broiler carcasses and of Methicillin-resistantStaphylococcus aureus(MRSA) in breeding pigs.The objectives of the studies are to generate comparableprevalence data from all Member States with the purposeof setting common EU targets for the reduction of thepathogen in question.In 2008, baseline studies onSalmonellaandCampylo-bacterprevalence in broilers and broiler carcasses and ofSalmonellaand MRSA in breeding pigs was carried outand the results are still being analysed.
Annual Report on Zoonoses in Denmark 2008
19
6. Surveillance and monitoring6.1 Surveillance of human diseasePresented in this report is the occurrence of zoonoticenteric pathogens in Denmark:• Notifiable through the laboratory surveillance system:Salmonella, Campylobacter, Yersinia,Verocytotoxin-producingE. coli(VTEC) andListeria• Individually notifiable zoonotic pathogens:Chlamydiapsittacci(ornithosis),Leptospira, Mycobacterium,Bo-vine Spongieform Encephalopathy (BSE) prions (var.Creutzfeldt-Jakob Disease), Verocytotoxin-producingE. coli(VTEC) andLyssavirus(rabies)• Non-notifiable zoonotic pathogens:Brucella, Crypto-sporidium, Echinococcus, ToxoplasmaandTrichinella.An overview of these notifiable and non-notifiablehuman diseases is provided in appendix D, Table A30.In Denmark, the physicians report individually notifia-ble zoonotic diseases to the medical officers and the De-partment of Epidemiology at Statens Serum Institut (Figure6.1). Positive cases diagnosed by a clinical microbiologicallaboratory are reported through the laboratory surveil-lance system to the Unit of Gastrointestinal Infections atStatens Serum Institut. Physicians send specimens fromsuspect cases to one of 15 clinical microbiology laborato-ries depending on county of residence of the requestingphysician. The laboratories must report positive results toStatens Serum Institut within one week. Furthermore, allSalmonellaand VTEC isolates are sent to the referencelaboratory at Statens Serum Institut for further sero- andgenotyping. TheSalmonellapositive isolates are sent tothe National Food Institute, Technical University of Den-mark for phage typing. See chapter 3 for more detailedinformation on typing methods. The results are recordedin the Register of Enteric Pathogens maintained by StatensSerum Institut. Positive cases are reported as episodes, i.e.each patient-infectious agent combination is only recorded
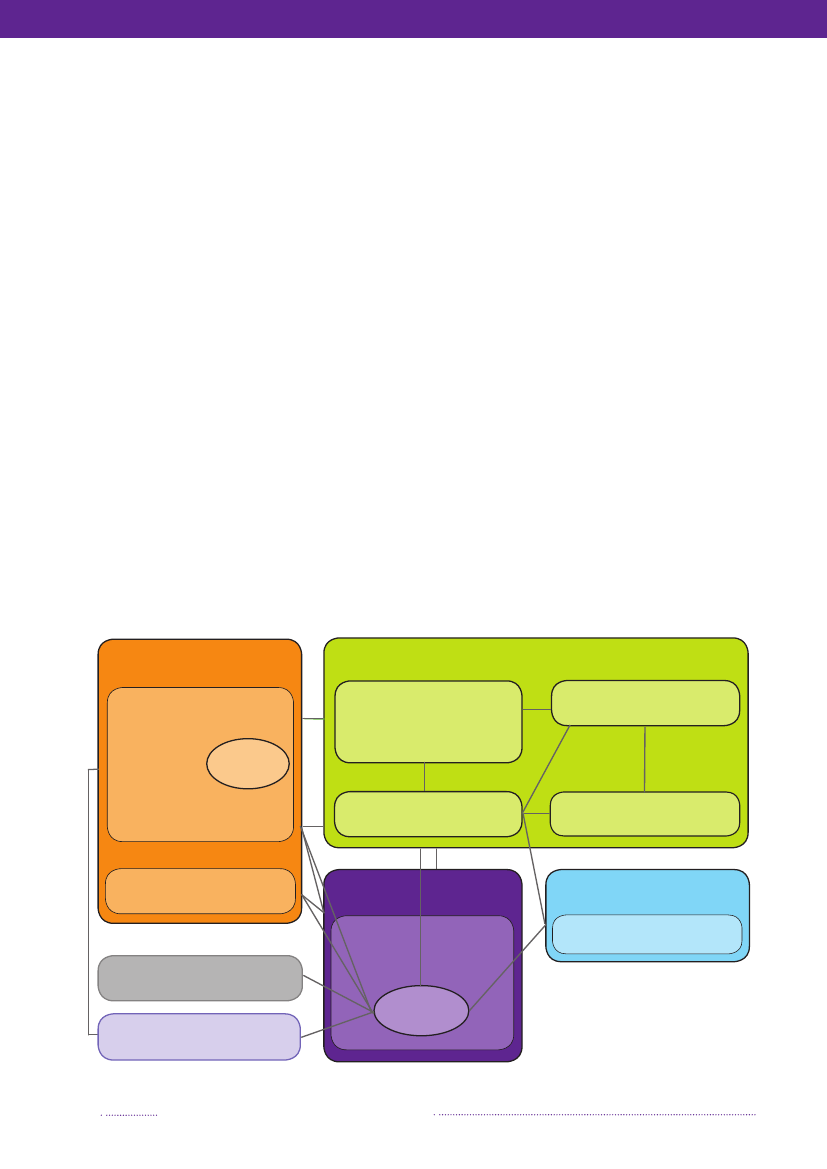
Figure 6.1. Overview of the monitoring and outbreak investigation network for reporting infectious patho-gens in humans, animals, foodstuffs and feedstuffs in DenmarkMinistry of Food,Agriculture and FisheriesDanish Veterinary and FoodAdministration (DVFA)Danish AlertUnit for Food
Ministry of the Interior and HealthNational Board of Health&5 Regional Medical Officers ofHealthGeneral Practitioners &Hospitals
&
3 Regional Veterinary &Food Control Authorities(RVFCA)
Statens Serum Institut (SSI)
Clinical MicrobiologyLaboratories
Danish Plant Directorate
Ministry of Science,Technology and InnovationTecnical University of Denmark(DTU)
Ministry of theEnvironmentDanish EnvironmentalProtection Agency
Industry
National Food InstituteDanish ZoonosisCentre
Non-governmentalOrganisation
Source: Danish Zoonosis Centre, National Food Institute, Technical University of Denmark
20
Annual Report on Zoonoses in Denmark 2008
once in any six-month period. Overviews of results fromthe Register of Enteric Pathogens are presented as follows:• All laboratory confirmed human cases are presented inappendix B, Table A2• Regional distribution of human cases of salmonellosisis presented in appendix B, Figures A1-A2• Regional distribution of human cases of campylobacte-riosis is presented in appendix B, Figure A3• Regional distribution of human cases of yersiniosis ispresented in appendix B, Figure A4• Regional distribution of human cases due to VTEC ispresented in appendix B, Figure A5.Further, additional information on human infectionsare presented as follows:• TheSalmonellasero- and phage type distributions arepresented in appendix C, Tables A5-A7• VTEC O-group distribution in humans is presented inappendix B, Table A3.
breaks Database (FUD) are presented in Appendix B, TableA4 and some of the more notable outbreaks are outlinedin Chapter 2.
6.3 Surveillance and monitoring of ani-mals and animal productsSurveillance and monitoring programmes for poultry,pigs and cattle are presented in Appendix D, Tables A32-A37. Sample analysis is performed at authorised privatelaboratories, the Regional Veterinary and Food ControlAuthorities, the National Food Institute or the NationalVeterinary Institute, Technical University of Denmark. Iso-lates positive withSalmonellaare forwarded to the NationalFood Institute for subtyping (sero-, phage and genotypingas well as antimicrobial susceptibility testing). See chapter3 for more detailed information on typing methods.Overviews of results from surveillance and monitoringofSalmonellaare presented as follows:• Results from the table-egg production are presented inappendix C, Tables A5-A9 and Figures A8-A9• Results from the broiler production are presented inappendix C, Tables A5, A7 and A10• Results from the duck and turkey produtions are pre-sented in appendix C, Table A14• Results from the pig production are presented in ap-pendix C, Tables A5-A7, A15 and Figures A6-A8• Results from the cattle production are presented in ap-pendix C, Tables A5, A7, A16-17 and Figure A9• Results from the feeding stuff production are presentedin appendix C, Tables A21• Results from the rendering plants are presented in ap-pendix C, Table A22• Results from pets, zoo animals and wild life are pre-sented in appendix C, Table A23.Salmonellasero- and phage type distribution in cattleand pig herds investigated due to clinical disease (not ne-cessarily salmonellosis) and found positive forSalmonellaare presented in appendix C, Table A18. Cattle herds withconfirmed infections of multiresistantS.TyphimuriumDT104 (MR DT104) or herds that have been in contactwith herds infected with MR DT104 are placed under of-ficial veterinary supervision. Cattle herds with confirmedinfection ofS.Dublin are subject to hygienic slaughter.Overviews of results from monitoring ofCampylobacterare presented as follows:• Results from the poultry production are presented inappendix C, Tables A11 and A13• Results from pig and cattle herds are presented in ap-pendix C, Tables A19• Results from pets, zoo animals and wild life are pre-sented in appendix C, Table A23.Pig and cattle carcasses are screened forMycobacteriumandEchinococcusduring meat inspection at the slaughter-Annual Report on Zoonoses in Denmark 2008
6.2 Outbreaks of zoonotic gastrointe-stinal infectionsIn Denmark, local foodborne outbreaks are typicallyinvestigated by the Regional Veterinary and Food ControlAuthority in collaboration with the medical officer; oftenwith the participation of the regional clinical microbiologylaboratory. Larger outbreaks involving more than one re-gion are typically investigated by Statens Serum Institut,the National Food Institute and the Danish Veterinary andFood Administration. These institutions may also aid inthe investigation of local outbreaks. Representatives fromthese institutions meet regularly to discuss surveillanceresults, compare the reported occurrence of zoonoticagents in animals, food and feedstuffs with that in humans,and review major outbreaks. The formal responsibility ofinvestigating food- or waterborne outbreaks is currentlydivided between three ministries based on the outbreaksource: the Ministry for the Interior and Health for in-fectious diseases; the Ministry of Food, Agriculture andFisheries for food and animal related diseases; and theMinistry of the Environment (along with the municipali-ties) for water related diseases.Outbreaks may be detected in various ways. Individualswho experience illness related to food intake in settingssuch as restaurants or work place cantinas may report theseincidents directly to the Regional Veterinary and FoodControl Authorities. Physicians are obligated to report allsuspected water- and foodborne infections to the regionalmedical officer, who then reports to Statens Serum Insti-tut. Clusters of cases may be noted in the laboratory oridentified at Statens Serum Institut through the laboratorysurveillance system of gastrointestinal bacterial infectionsor through subtyping of bacterial isolates from patients.A list of verified outbreaks (not including householdoutbreaks) reported to the Food- and waterborne Out-
21
Surveillance and monitoring
Changes to theSalmonellasurveillance programme for broiler flocksIn 2008, theSalmonellasurveillance programme for broiler flocks was changed; one additional AM-testing of broiler flocks was introduced 7-10 days prior to slaughter in addition to the samples alreadycollected 2-3 weeks before slaughter. The results of the tests must be available before slaughter. Allflocks are sampled, also flocks intended for export before slaughter.From mid 2008, all AM-positive flocks were slaughtered at one slaughterhouse in Denmark and specialhygienic precautions are in action at slaughter; all positive flocks are heat treated and 300 samples arecollected from the flock thereafter.Surveillance ofSalmonellain broiler flocks is carried out according to Order no 1261 of 15/12/2008,and further information on the surveillance programme are given in appendix D, table A33.house. Although Denmark is assigned as a region wherethe risk ofTrichinellain domestic swine is negligible (seeparagraph 5.1), all slaughter pigs slaughtered at export ap-proved slaughterhouses are still examined forTrichinellaaswell as all horses slaughtered for human consumption andall wild boars. In addition, boars and bulls are tested forBrucellaand bulls are tested forMycobacteriumat semencollection centres. All positive results for notifiable infec-tious diseases are reported to the Danish Veterinary andFood Administration. Results are presented in appendixC, Table A15-A16.Results from the surveillance for Bovine SpongiformEncephalopathy (BSE) in cattle, Transmissible SpongiformEncephalopathy (TSE) in sheep/goats and Chronic WastingDisease (CWD) in deer are presented in appendix C, TablesA24-A25 and A27.Results from the monitoring ofCoxiella brunetii(Qfever) in cattle are presented in appendix C, Table A14.Appendix D, Table A31 gives an overview of notifiableand non-notifiable zoonoses presented in this report alongwith the relevant legislation.high risk products, specific types of production proces-ses or specific types of food establishments• Other types of sampling at the food wholesale andretail level include:* Sampling based on suspicion to support findingsfrom inspection of food establishments* Sampling at the wholesale level to verify compli-ance with microbiological criteria in the legislation* Sampling in relation to the investigation of food-borne outbreaks* Sampling in response to consumer complaints.Centrally co-ordinated control is carried out as nationalprojects or surveys. The purposes of these projects are to:• Verify compliance with microbiological criteria laiddown in the legislation• Discover emerging problems with microbiologicalcontaminants• Generate data for the preparation of risk profiles andrisk assessments to support microbial risk management• Monitor the effect of established risk management pro-cedures in order to evaluate if these provide the desiredresults or if they need to be reconsidered.Appendix C, Table A28 provides information on thecentrally co-ordinated projects conducted in 2008. Infor-mation on the following projects is presented:• The intensified control ofSalmonellaandCampylobacterin Danish and imported meat are presented in AppendixC, Table A20• The findings ofCampylobacterin non-heat treated meatcuts from broilers are presented in Appendix C, TablesA11 and A12• Findings ofListeria monocytogenesin ready-to-eat pro-ducts are presented in Appendix C, Table A29.For further information consult the webpage of theDanish Veterinary and Food Administration, www.fvst.dk (in Danish).
6.4 Official testing of zoonotic patho-gens in foodstuffsIn Denmark, control of pathogens in foodstuffs iscoordinated both at the regional and at the central levelof administration. Each Regional Veterinary and FoodControl Authorities is responsible for the control carriedout within its own region, and the Danish Veterinary andFood Administration is responsible for the regulation,control strategy and the surveillance at the national level.The main purpose of the regional microbiologicalcontrol system is to verify that the own-check program-mes implemented at food establishments are functioningeffectively and to verify the compliance with the microbio-logical criteria laid down in the legislation.Regional microbiological control is carried out as fol-lows:• Targeted survey sampling primarily at the retail level.These surveys are focused on collecting samples from
22
Annual Report on Zoonoses in Denmark 2008
Surveillance and monitoring
Antimicrobial ResistanceFor information on antimicrobial resistancein zoonotic bacteria please refer to the annualreport ”DANMAP – Consumption of antimicrobialagents and occurrence of antimicrobialresistance in bacteria from food animals, food andhumans in Denmark”. The 2008 DANMAP reportis available from www.danmap.org or may beordered from the Danish Zoonosis Centre([email protected]).
Annual Report on Zoonoses in Denmark 2008
23
Appendix ATrends and sources in human salmonellosis
Table A1. Estimated no. of reported human cases and percentage of cases per major food source, travel oroutbreaks, 2006-2008200820072006Estimated no. ofEstimated no. ofEstimated no. ofPercentagePercentagePercentagereported casesreported casesreported casesof reportedof reportedof reportedSource(95% credibility(95% credibility(95% credibilitycasescasescasesintervalb)interval)interval)Pork320 (277-367)8.8107 (59-159)6.5101 (77-129)6.1Beef26 (16-36)0.712 (2-27)0.823 (12-33)1.4Table eggs116 (91-143)3.2181 (147-217)11.0103 (81-124)6.2Broilers47 (25-133)1.312 (2-30)0.88 (3-16)0.5Ducks38 (2-99)1.0--12 (3-23)0.7Imported pork39 (12-70)1.121 (4-46)1.326 (12-43)1.6Imported beef12 (3-25)0.320 (10-29)1.222 (12-34)1.3Imported chicken191 (120-250)5.261 (34-87)3.7152 (123-184)9.2Imported turkey87 (8-151)2.412 (2-29)0.787(67-108)5.2Imported duck----11 (5-20)0.7853 (843-864)23.3762 (731-794)46.341024.7Travels1Unknown source480 (413-547)13.1386 (329-441)23.4605 (556-653)36.5Outbreaks, unknown1,44739.6734.4985.9sourceTOTAL3,6561,6471,6581) The estimate of travel related cases should be interpreted carefully, since availability of travel history data was incompletefor 2006.2) The model is based on a Bayesian framework which gives 95% credibility intervals.Source: Danish Zoonosis Centre, National Food Institute, Technical University of Denmark
24
Annual Report on Zoonoses in Denmark 2008
Appendix BHuman disease and outbreak data
Table A2. Zoonoses in humans, number of laboratory-confirmed cases, 1999-2008IncidenceReported no. of casesper 100,00020082008 200720062005 2004Zoonotic pathogenBacteriaBrucella abortus/melitensisa,c-8209154bCampylobacter coli/jejuni63.13,454 3,868 3,242 3,671 3,724bChlamydia psittaci0.16117228bLeptospiraspp.0.21310152433bListeria monocytogenes0.95158564641Mycobacterium bovisb0.011130266.83,656 1,647 1,658 1,775 1,538SalmonellabS.Enteritidisb11.7638566562642546S.Typhimuriumb36.62,002343411565467Other serotypesb18.61,016740687568525VTEC totalb2.9161161146154168O1570.31525192547other or non-typeable2.6143136127129121bYersinia enterocolitica6.0330270215241228ParasitesCryptosporidiumspp.a,c-9249---a,eE. multilocularis-03---a,e-59---E. granulosusa,fToxoplasma gondii---1498a,c,eTrichinellaspp.-01---VirusesLyssavirusb-00000
1999-4,164-234423,2682,025584659511041339----
0
a) Not notifiable hence the incidence cannot be calculated.b) Notifiable.c) Data presented are from one laboratory (Statens Serum Institute) only, representing a proportion of theDanish population (approximately 1/3 in 2008). The proportion of the population represented varies from yearto year, thus results from different years are not comparable. Testing for these pathogens is carried out only ifspecifically requested on the submission form.e) The cases were imported.f) The nation-wide neonatal screening for congenital toxoplasmosis stopped in 2007.Source: Statens Serum Institute
Annual Report on Zoonoses in Denmark 2008
25
Appendix B
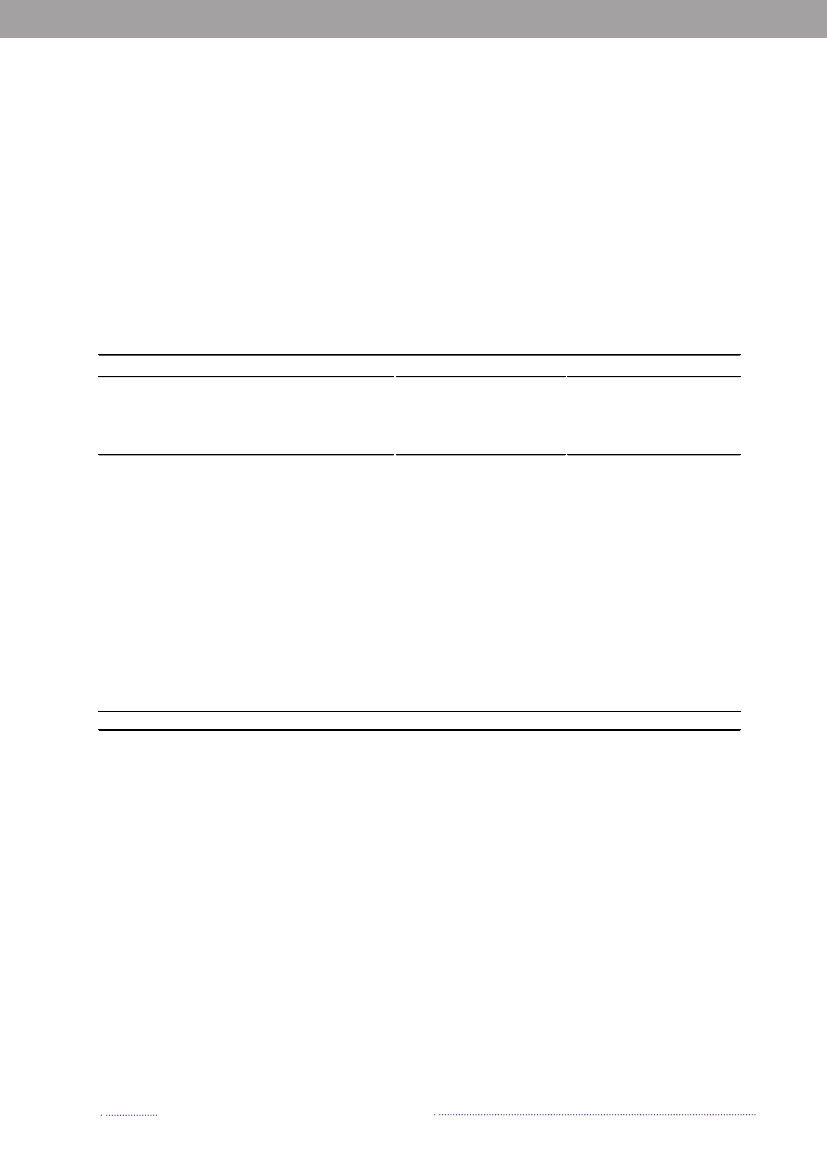
Figure A1. Geographical distribution of human cases per county and incidence of human infections withS.Enteritidis, 2008063
267029366022572715357890
30Incidence of S. Enteritidis (cases per 100,000)
Source:Statens Serum Institut
0 - <5
5 - <10
10 - <15
15 - <20
Figure A2. Geographical distribution of human cases per county and incidence of human infections withS.Typhimurium, 2008
2157
14124312791138942441019791186 215
76Incidence of S. Typhimurium (cases per 100,000)< 3030 - < 4050 - <5050 - < 60
Source:Statens Serum Institut
26
Annual Report on Zoonoses in Denmark 2008
Appendix B
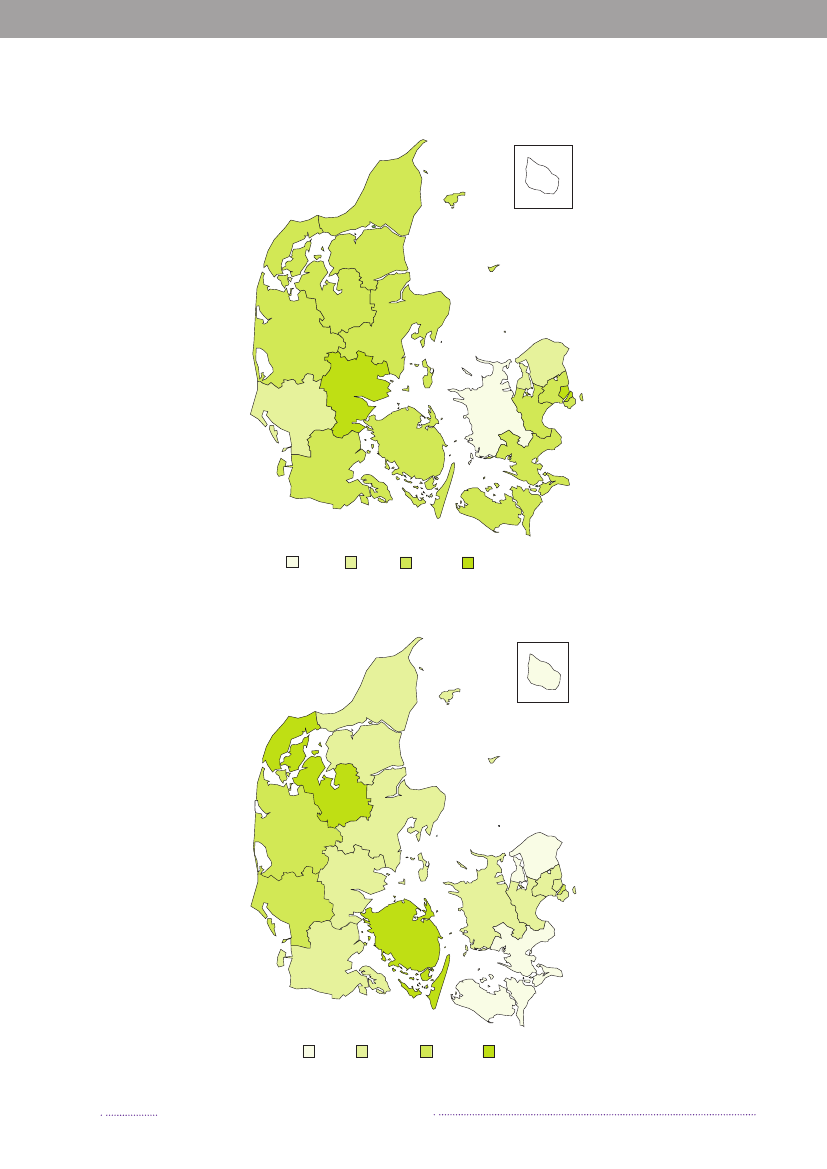
Figure A3. Geographical distribution of human cases per county and incidence of human infections withCampylobacter,2008
12231
126315217264188185345152130182408525
163Incidence of Campylobacter (cases per 100,000)0 - <40
Source:Statens Serum Institut
40 - <60
60 - <80
80 - <100
Figure A4. Geographical distribution of human cases per county and incidence of human infections withYersinia,2008
126
93423712198409133052
9Incidence of Yersinia (cases per 100,000)0 - <3.03.0 - <6.06.0 - <9.0>= 9.0
Source:Statens Serum Institut
Annual Report on Zoonoses in Denmark 2008
27
Appendix B
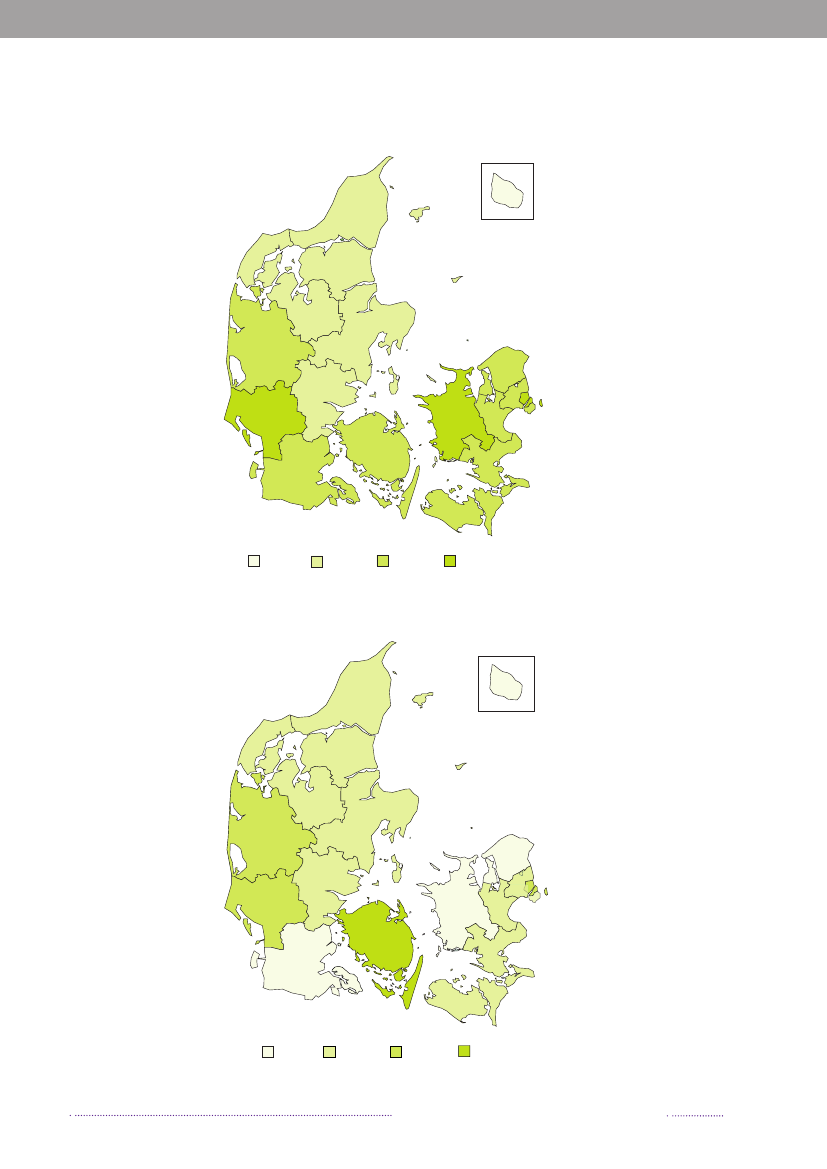
Figure A5. Geographical distribution of human cases per county and incidence of human infections withVTEC,2008. The circled counties offer testing by molecular detection
07
11421905352315542
6Incidence of VTEC (episodes per 100,000)0-<22-<44 <66-<8
Source:Statens Serum Institut
Table A3.VTECO-group distribution inhumans, 2008Number ofO-groupaepisodesO103O157O146O91O145O111O117O128abO26O121O- roughOther O-groups or not-typedTOTAL191512111198765649158
a) All O-groups that resulted in five or moreepisodes are listed.Source:Statens Serum Institut
28
Annual Report on Zoonoses in Denmark 2008
Appendix B
Table A4. Foodborne disease outbreaksareported in the Food- and waterborne Outbreak Database(FUD), 2008PatientsNo. ofPathogenlaboratorySettingSuspected sourcepatientsconfirmedCucurbitacin10.HotelComposite mealCucurbitacin4.FarmFresh vegetablesHistamin2.ShopFishHistamin2.Restaurant/cateringFishNorovirus112OtherUnknownNorovirus70Restaurant/cateringUnknownNorovirus36.CanteenUnknownNorovirus24.Private partyBuffet mealsNorovirus22.HospitalUnknownNorovirus1146CanteenBuffet mealsNorovirus16.ShopComposite mealNorovirus913Restaurant/cateringBuffet mealsNorovirus18.Restaurant/cateringBuffet mealsNorovirus3.Restaurant/cateringComposite mealNorovirus205InstitutionBuffet mealsNorovirus24.CanteenComposite mealNorovirus30.Restaurant/cateringBuffet mealsNorovirus6.CanteenBuffet mealsNorovirus27.CanteenBuffet mealsNorovirus66.Restaurant/cateringBuffet mealsNorovirus232Restaurant/cateringBuffet mealsNorovirus276Restaurant/cateringBuffet mealsNorovirus284HotelBuffet mealsNorovirus326Restaurant/cateringComposite mealNorovirus16.ShopComposite mealNorovirus52Restaurant/cateringComposite mealNorovirus68.CanteenUnknownNorovirus13.HotelUnknownNorovirus22.HotelUnknownNorovirus3212Restaurant/cateringUnknownCalcivirus (not norovirus)154CanteenBuffet mealsVirus15.InstitutionUnknownVirus11.Restaurant/cateringUnknownStaphylococcus aureus42.OtherComposite mealShigella flexneri174Private partyUnknownSalmonella43.Private partyUnknownS. Agona44CanteenComposite mealS. Agona.10General outbreakUnknownS. Enteritidis408Private partyUnknownS. Enteritidis..Tourists from GreeceUnknownS. Derby.10General outbreakPorkS. Derby.22General outbreakUnknownS. Kottbus.5General outbreakUnknownContinued…
FUDno.811835834805814791819830787850849847846842841839838837833829803801800780779806883882879867799820786881795809810859873861875874784
Annual Report on Zoonoses in Denmark 2008
29
Appendix B
Table A4. Foodborne disease outbreaksareported in the Food- and waterborne Outbreak Database (FUD),2008 (Continued from page 29)PatientsNo. ofFUDPathogenlaboratorySettingSuspected sourcepatientsno.confirmedS. Typhimurium U312b.24SlaughterhousePork863S. Typhimurium U288.39Food producerPork855bS. Typhimurium DT 135.109General outbreakUnknown854bS. Typhimurium DT 3.61General outbreakUnknown853S. Typhimurium DT 120.53ShopPork (ham)852S. Typhimurium U288.37Restaurant/cateringShawarma793bS. Typhimurium U292.1,224General outbreakUnknown788S. Stanley.5General outbreakFresh vegetables860S. Chester98General outbreakUnknown796Clostridium perfringens58.CanteenBuffet meals812Clostridium perfringens191Restaurant/cateringFresh vegetables831Clostridium perfringens35.Private partyComposite meal823Clostridium perfringens5.Restaurant/cateringOther foods802Campylobacter51Restaurant/cateringChicken845Campylobacter2811Tourists from IndiaChicken781Bacillus cereus23.CanteenComposite meal840Bacillus cereus4.Restaurant/cateringComposite meal825Bacillus cereus10.Restaurant/cateringOther meat778Listeria monocytogenes32OtherOther meat878Unknown26.InstitutionComposite meal818Unknown6.Restaurant/cateringUnknown807Unknown16.HotelUnknown827Unknown150HotelBuffet meals797Unknown2.CanteenComposite meal792Unknown20Restaurant/cateringComposite meal777Unknown22.Private partyUnknown783Total1,2741,690a) In addition, 14 confirmed household outbreaks were registered. These were caused byCampylobacter(3 outbreaks),S.Infantis(1), Norovirus (2),Clostridium perfringens(1), Cucurbitacin (squash 1), Histamin (1) and unknown pathogens (5).b) The outbreak continued in 2009, only cases from 2008 are reported here.
30
Annual Report on Zoonoses in Denmark 2008
Monitoring and surveillance dataTable A5. Serotype distribution (%) ofSalmonellafrom humans, animals, carcasses at slaughterhouse andimported meat, 2008PigCattleLayer Broiler DuckImported meatfHuman herdsaPorkbherdscBeefbflocksdflockseflocksePork Beef Chicken TurkeySerotypeN=3,656 N=554 N=199 N=33 N=11 N=4 N=43 N=76 N=61 N=6 N=110 N=66Typhimurium54.870.444.2 60.69.125.025.63.954.116.77.324.2Enteritidis17.500.50025.001.30010.01.5Agona1.90.41.000000008.20Newport1.6000050.001.30006.1O:4,5,12; H:i:-1.600000000000Derby1.519.327.60004.7016.402.76.1Stanley1.200000000000Java1.100000000000Infantis1.03.46.50009.303.305.50Saintpaul1.00000000001.836.4Others16.86.520.1 39.490.9060.593.426.283.364.525.8TOTAL100100100100100100100100100100100100a) Isolates obtained from sampling of herds placed in level 2 and 3 (See Table A37 for detailes on the surveillance programme).b) Swab samples of pork and beef carcasses from the surveillance programme at slaughterhouses.c) Cattle herds examined based on clinical indication. The data are not representative for the Danish cattle population.d) Represenative samples from the surveillance programme in prodution flocks.e) Representative faecal or sock samples from the mandatory AM inspection prior to slaughter.f) Case-by-case monitoring of imported meat and meat products.Source: Danish Veterinary and Food Administration, Statens Serum Institut and National Food Institute, Technical University ofDenmark
Appendix C
Table A6. Phage type distribution (%) of S. Enteritidisafrom humans, animals and im-ported meat, 2008Imported meatfHumanLayer flocksdChickenTurkeyPorkbn=638n=1n=1n=11n=1PT 824.110010000PT 2116.50000PT 14B12.90000PT 110.80018.20PT 48.30054.5100PT RDNC7.1009.10PT 63.1009.10PT 6A2.80000PT 1B2.00000PT 13A1.90000Others10.5009.10Total100100100100100
a) The total number of samples may differ between phage type and serotype tabels (Tables A5-A7), sinceisolates of one serotype may contain more than one phage type.b, d and f): See Table A5.Additionally, one isolate from a turkey flock and one isolate from a duck flock were positive withS.Ente-ritidis.Source: Danish Veterinary and Food Administration, Statens Serum Institute and National Food Institute,Technical university of Denmark
Annual Report on Zoonoses in Denmark 2008
31
Appendix C
Appendix C
Table A7. Phagetype distribution (%) of S. Typhimuriumgfrom humans, animals and importedmeat, 2008PigLayers Broiler DuckImported meatfCattleHuman herdsaPorkbherdscBeefbflocksdflockseflocksePorkBeefChickenPhagetypen=2002 n=390 n=95 n=20n=1n=1 n=11n=3 n=33n=1n=8U29251.00015.00018.20000DT 1206.020.018.90009.1012.11000DT RDNC6.07.94.2000003.03050.0DT 1354.91.52.100000000U2883.02.11.100000000DT 32.70000000000DT 1932.56.72.10000024.2025.0DT 121.814.615.85.00027.30000U3021.72.68.400000000DT 104+104B1.712.18.425.0000021.2012.5Others18.632.638.955.010010045.510039.4012.5Total100100100100100100100100100100100
Turkeyn=16012.56.300031.30037.512.5100
a-f) See Table A5.g) The total number of samples may differ between phage type and serotype tabels (Tables A5-A7), since isolates of one serotypemay contain more than one phage type.Source: Danish Veterinary and Food Administration, Statens Serum Institut and National Food Institute, Technical Universityof Denmark
Table A8. Occurrence ofSalmonellain the table egg productiona, 2000-2008Rearing periodProduction periodRearing flocks(parent flocks)(parent flocks)NPositiveNPositiveNPositive20001502903748200114022033942002150220330920032401503674b200492903681200516090255620061701102892200711012032602008100602581c
Layer flocksN688607619611641655565510508Positive3235151057254d
a) See Tables A32 and A34 for describtion of the surveillance programmes.b) Two positive flocks in the same holding; the second flock was registered approx. six weeks after the first flock.c)S.Typhimurium DT 7.d) One battery flock positive withS.Enteriditis PT 8, one organic flock positive withS.Typhimurium DT 41 and two freerange flocks positive withS.Newport (same holding and same house found twice in 2008).Source: Danish Veterinary and Food Administration
32
Annual Report on Zoonoses in Denmark 2008
Appendix C
Table A9. Occurrence ofSalmonellain the table egg layer flocks according to type of production,2000-2008Deep litterFree rangeOrganicBatteryNPositiveNPositiveNPositiveNPositive200086048511197916200112224616137312914200212314941304127720031912712173116792004214072217511772200521737001780175420061850620164214802007155256014621461ab2008151061214511351ca) Two flocks positive withS.Newport - same holding and same house found twice in 2008.b) One flock positive withS.Typhimurium DT 41.c) One flock positive withS.Enteritidis PT 8.Source: Danish Veterinary and Food Administration
Table A10. Occurrence ofSalmonellain the broiler production, 2000-2008SlaughterhouseRearing periodProduction periodBroiler flocks(flocks/batches)(parent flocks)(parent flocks)NNNNPositivePositivePositivePositive2000222334534,500954,543131a2001243032574,571761,695692002241233024,443681,66792c2003265218244,414771,55277c2004275115564,246641,47224c2005214018504,034871,174272006190028253,62171775b172007152025833,7366082810200814602932d3,71743518e3a) PM sampling at the slaughterhouse were changed from pooled neck skin samples of flocks to chicken cutssampling of batches.b) From 2006, data cover only samples taken following theSalmonellaprogramme in 2006. Verification samp-les taken once a week by producers of poultry meat approved to marketSalmonella-freepoultry meat are notincluded, this sampling started in middle of 2005.c) In 2003-2005, only one flock per house was registered per year although there may have been more than oneflock in the house, however all flocks were sampled according to the surveillance programme.d) One flock positive withS.Typhimurium DT 41, one withS.Typhimurium DT 120.e) From 2008, all AM positive flocks are heat treated at slaughter. Sampling is now carried out as verification ofthe AM results of the negative flocks. See Tables A32 and A33 for describtion of the surveillance programmes.Source: Danish Agriculture and Food Council and Danish Veterinary and Food Administration
Annual Report on Zoonoses in Denmark 2008
33
Appendix C
Appendix C
Table A11. Occurrence ofCampylobacterin broiler flocks and in fresh meat at slaughter, 2000-2008YearBroiler flocksaChilled broiler meatbN% posN% pos20006,14637.6--20016,05441.8--20026,20842.6--20035,37334.2--20045,15727.01,60317.820054,95230.41,68912.320064,52230.89597.920074,52726.84398.2c20084,91225.948414.7c
a) Flocks investigated by cloacal swabs collected at slaughter, samples are pooled and analysed as one sample using PCR.b) Centrally co-ordinated studies, slaughterhouse samples (see section 6.4 for describtion). Detection limit <10 cfu/g.c) Data are not compareable to previous years as they only represent the last two quarters of the year, which is the high prevalentperiod.Source: Danish Veterinary and Food Administration, Danish Agriculture and Food Council and National Veterinary Institute,Tecnical University of Denmark
Table A12. Occurrence ofCampylobacterin non-heat treated broiler meat at retaila,b, 2001-2008Chilled broiler meat (samples)Frozen broiler meat (samples)YearDenmarkImportDenmarkImportNNNN% pos% pos% pos% pos(adjusted)(adjusted)(adjusted)(adjusted)2001-200276236.719055.048515.621534.52002-200340340.813978.532418.316724.92003-200433427.217065.756610.927219.62004-200551731.129973.293712.239125.92005-200640129.885456.31,08713.569831.32006-200736331.01,12851.189719.081233.92007-20081,05932.91,06753.965529.657744.4a) Centrally co-ordinated studies, retail samples (see section 6.4 for describtion). 2000-2002: detection limit <0.4 cfu/g; 2003-2008: detection limit <0.1 cfu/g.b) The prevalence is calculated as a mean of quarterly prevalences based on the sum of data from the two years specified.Source: National Food Institute, Technical University of Denmark
Table A13. Relative distribution ofCampylobacterspecies (%) in broilers before slaughtera, 2002-2008C. jejuniC. upsaliensisC. coliNT/otherYearN% pos% pos% pos% pos200217893.306.70200311392.906.20.9b200410194.105.90200510990.82.806.4200611392.00.97.10200711191.95.40.91.8200810090.52.80.06.6a) Positive isolates collected as part of the DANMAP programme was examined using conventional microbiological methods.b)C. lari.Source: National Veterinary Institute, Technical University of Denmark
34
Annual Report on Zoonoses in Denmark 2008
Appendix C
a) See Table A35 for description of the surveillance programmes.b) The two major turkey and duck slaughterhouses in Denmark closed down in 2004 and 2007, respectively.Therefore, most commercially raised duck and turkey flocks are transported abroad for slaughter.c) Not available.Source: Danish Agriculture and Food Council
Table A14. Occurrence ofSalmonellain turkey and duck flocksa,b, 2006-2008Duck flocksTurkey flocksYearN% posN200625581.232c2007n.a.n.a.n.a.20086170.569
% pos0n.a.1.4
Figure A6. Serological surveillance ofSalmonellain breeding and multiplying pigs basedon monthly testing of blood samples, 2003-2008. For more information about the surveil-lance programme, see table A37
8
% positive breeding- andmultiplying pigs
6420Jan 2003 Jan 2004 Jan 2005 Jan 2006 Jan 2007 Jan 2008% positive% positive, moving avg. for 12 month
Source: Danish Agriculture and Food Council
Figure A7. Serological surveillance ofSalmonellain slaughterpigs, 2003-2008. Percentageof seropositive meat juice samples (first sample per herd per month). The abrupt incre-ase in 2003 was attributed, in part, to analytical-technical adjustments. The peak in latesummer 2007 and the very low level during 2008 were due to technical problems in thelaboratory. For more information about the surveillance programme, see table A37
18
% positive slaughter pigs
15129630Jan 2003 Jan 2004 Jan 2005 Jan 2006 Jan 2007 Jan 2008% positive% positive, moving avg. for 12 monthAnnual Report on Zoonoses in Denmark 2008
Source: Danish Agriculture and Food Council
35
Appendix C
Appendix C
Table A15. Occurrence of zoonotic pathogens in pigs and pork in Denmark, 2008SlaughterhousePrimary production(slaughtering >50pigs per month)HerdsZoonotic pathogenSalmonellaaBrucella abortuseMycobacterium bovisfEchinococcusfgranulosis/multilocularisLeptospiragTrichinellaspp.hN9,445---26-b
Slaughterhouse(slaughtering 50 orless pigs per month)Samples
Herds/AnimalsPositive18500000b
Animals/samplesN-24,79618,582,28818,582,2886918,582,288
SamplesN27,045-----c
% pos1.3-----d
N144-----c
% pos2.0d-----
a) See Table A37 for describtion of the surveillance programme.b) Data are from December 2008. Slaughterpig herds monitored using serological testing of meatjuice samples collected at slau-ghter. Herds belonging to level 2 and 3 were defined asSalmonellapositive.c) Swabs from three areas of the half-carcass were collected at the slaughterhouse after min. 12 h chilling. Sample size is 3x100cm2. Samples from 5 animals were pooled, except at slaughterhouses where 50 pigs or less were slaughtered per month, in whichcase samples were analysed individually.d) When estimating the prevalence ofSalmonella,both the loss of sensitivity and the probability of more than one sample beingpositive in each pool are taken into consideration. A conversion factor has been determined on the basis of comparative studies,as described in Annual Report 2001.e) Including samples from boars (examined at pre-entry, every 18 month, and prior to release from semen collection centres)(15,739 samples), samples collected in connection with export (8,790 samples), import (124 samples) or fertility problems (143samples). 5-8 ml blood samples were analysed using either the SAT, RBT or CFT methods.f) Slaughtered pigs were examined by slaughterhouse meat inspectors.g) Sampling is based on suspicion of leptosporosis due to increased abortions or other reproductive problems in a herd. Samplesare investigated using immunoflourescence techniques.h) Samples from all pigs slaughtered at export approved slaughterhouses were examined using the method described in Directive2075/2005/EEC. In 2007, Denmark achieved official status as region with negligible risk ofTrichinella,according to EU Regula-tion (EC) No 2075/2005.Source: Danish Veterinary and Food Administration, National Veterinary Institute and National Food Institute, Technical Uni-versity of Denmark
Figure A8.Salmonellain pork, monitored at slaughterhouses, 2003-2008. Swab samples from threedesignated areas of chilled half-carcasses
2.0
% positive samples
1.51.00.50.0Jan 2003 Jan 2004 Jan 2005 Jan 2006 Jan 2007 Jan 2008% positive% positive, moving avg. for 12 month
Source: Danish Veterinary and Food Administration
36
Annual Report on Zoonoses in Denmark 2008
Appendix C
Table A16. Occurrence of zoonotic pathogens in cattle and beef in Denmark, 2008Slaughterhouse(slaughtering >50Primary productioncattle per month)HerdsZoonotic pathogenSalmonellaa,bBrucella abortusc,dMycobacterium bovise,fEchinococcususfgranulosis/multilocularisCoxiella brunetiiN-----607hHerds/AnimalsPositive-00026362Animals/SamplesN-2,994511,300511,300229g-SamplesN7,915-----% pos0.2-----
Slaughterhouse(slaughtering 50 orless cattle per month)SamplesN205-----% pos0-----
a) Swabs from three areas of the half-carcass were collected at the slaughterhouse after min. 12 h chilling. Sample size is 3x100cm2. Samples from 5 animals were pooled, except at slaughterhouses where 50 cattle or less were slaughtered per month, in whichcase samples were analysed individually. See Table A36 for describtion of the surveillance programme.b) When estimating the prevalence ofSalmonella,both the loss of sensitivity and the probability of more than one sample beingpositive in each pool are taken into consideration. A conversion factor has been determined on the basis of comparative studies,as described in Annual Report 2001.c) Denmark has been declared officially brucelosis free since 1979. The last outbreak was recorded in 1962.d) Including samples from boars (examined at pre-entry, every 18 month, and prior to release from semen collection centres)(2.627 samples), samples collected in connection with export (225 samples) or fertility problems (142 samples). 5-8 ml bloodsamples were analysed using either the SAT, RBT, CFT or ELISA methods.e) Denmark has been declared officially tuberculosis free since 1980. The last case of TB in cattle was diagnosed in 1988.f) Slaughtered cattle were examined by the slaughterhouse meat inspectors.g) Serum samples taken for diagnostic testing and analysed using an ELISA method. An additional 25 samples from placenta wasanalysed using the FISH method, 4 samples were positive.h) Bulk tank milk samples taken for diagnostic testing and analysed using an ELISA method.Source: Danish Veterinary and Food Administration, National Veterinary Institute and National Food Institute, Technical Uni-versity of Denmark
Figure A9.Salmonellain beef, monitored at slaughterhouses, 2003-2008. Swab samples taken from threedesignated areas of chilled half-carcasses
1.5
% positive samples
1.00.50.0200320042005200620072008
% positiveSource: Danish Veterinary and Food Administration
% positive, moving avg. for 12 month37
Annual Report on Zoonoses in Denmark 2008
Appendix C
Appendix C
Table A17. Cattle herds assigned to level 1-3 according to theS.Dublin surveillance programmea,January 2009Non-milkMilk producingSalmonellaDublin levelproducing herdsherdsN% posN% posLevel 11a1bTotalLevel 2222TotalLevel 3TotalUnknownTOTALTiter high in blood- or milk samplesContact with herds in level 2 or 3'Non-Level 1' due to too few blood samplesNonSalmonellaDublin freeSalmonellosis, official supervisionToo few blood samples56196591,535279815,9953.56.00.19.6<0.15.0594395638604,42213.40.90.114.40.10On the basis of milk samplesOn the basis of blood samplesProbablySalmonellaDublin free1,08412,57613,6606.878.685.43,753253,77884.90.685.4
a) See Table A36 for describtion of the surveillance programme.Source: Danish Veterinary and Food Administration
Table A18. Isolation ofSalmonellafrom outbreaks of clinicaldisease in pig and cattle herds, 2008Pigs herdsCattle herdsSerotype and phage typeS.Dublin13S.Typhimurium110S.Typhimurium DT10425S. Typhimurium DT121S. Typhimurium DT10711S. Typhimurium DT1931S. Typhimurium U2923TOTAL533Source: Danish Veterinary and Food Administration
38
Annual Report on Zoonoses in Denmark 2008
Appendix C
Table A19. Distribution ofCampylobacter(%) in pig and cattle herdsa, 2000-2008PigsCattle% pos% posNNC. coliC. jejuniother/unknownC. coliC. jejuniother/unknown200031059.44.20.6901.156.73.3200123868.52.95.5766.653.911.8200224078.81.70.087063.22.32003259--93.488--63.6200419178.01.00.5671.562.70200518583.22.2073042.50200629550.81.402246.737.50200726176.61.901323.067.40b200829266.11.7-1683.058.3-a) Samples were collected as part of the DANMAP programme. Caecal content was tested from one animal per herd.b) In 2008, samples were only tested forC. coliandC. jejuni.Source: National Food Institute, Tecnical University of Denmark
Table A20. Results from the intensified control ofSalmonellaandCampylobacterin fresh meatbased on a case-by-case risk assessment, 2008No. of batchestestedCampylobacterDanishPoultryImported PoultrySalmonellaBeefPorkPoultryImported BeefPorkPoultryDanish31093831831031013749093841192938135314321391303155030.9%32.2%10.3%17.3%1.7%36.1%6.9%18.6%2.62.8110.512.20.0495.44.82.3MeanNo. of batches No. of batches prevalence inpositivepositivesanctionedbatchesa,bMean relativehuman risk inpositivebatchesa
a) Include positive batches where a risk assesments has been performed. Risk assesments of positive batches ofmarinated meat is not required, but conducted in most cases.b) TheSalmonellaprevalence in each batch is based on the proportion of positive pooled samples (12 pools perbatch) and number of subsamples per pool.Source: Danish Veterinary and Food Administration and National Food Institute, Technical University of Denmark
Annual Report on Zoonoses in Denmark 2008
39
Appendix C
Table A21. Control ofSalmonellain compound feeds, feed processing and feed material, 2004-200820082007200620052004SamplesSamplesSamplesSamplesSamplesN PositiveN PositiveN PositiveN PositiveN PositiveFeed processing plants(process control)a:Ordinary inspections 1,085Additional inspectionsFeed materials, farm174animalsbTransport vehicles,hygiene samplesc318d976-719517-301,58917433619131131621,8851751,11925429157232,0081561,1273173021493
12e0
a) The presence ofSalmonellain compound feed is indirectly monitored by the environmental samples collected during feedprocessing. Companies are sampled one to four times per year.b) Sampling of feed materials used without further heattreatment (predominantly soy bean meal and rapeseed cake).c) Samples from transport vehicles (hygiene samples) prior to loading of feed compounds.d) 1S.Agona, 5S.Infantis, 1S.Isangi, 1S.Kralingen, 1S.Lexington 15,34, 2S.Liverpool, 2S.Mbandaka, 1S.Montevideo, 1S.Ruiru and 3S.Senftenberg.e) 3S.Agona, 1S.Cubana, 1S.Infantis, 1S.Kralingen, 1S.Lexington, 1S.Livingstone, 1S.Mbandaka and 3S.Senftenberg.Source: Danish Plant Directorate
Table A22. Three categories of meat and bone meal by-products not intended for human consumptiona,2008Category ofprocessing plant1By-products of this material cannot be used forfeeding purposes23By-product of this material may be used for feedfor fur animalsBy-products from healthy animals slaughteredin a slaughterhouse. Products of these may beused for petfood and for feed for fur animalsTOTALa) Regulation No. 1774 of 03/10/2002.Source: Danish Veterinary and Food Administration
Own-check samples Product samplesNPosNPos----517501
1,0851,085
1111
2,3892,569
6667
40
Annual Report on Zoonoses in Denmark 2008
Appendix C
Table A23. Occurrence of zoonotic pathogens in pets, zoo animals and wild life in Denmarka, 2008Pet animalsDogsZoonotic pathogenSalmonellaCampylobacterspp.Brucella canis/abortusChlamydia psittaciCryptosporidiumspp.Echinococcusspp.Trichinellaspp.kEuropean BatLyssavirusN101931036000pos000-7---CatsN414019000pos11-01---OthersN05041000pos-0-01d---Zoo animalsMammalBirds& reptilesN191075035040pos2g3b0-6e-0-N1500460000pos1h0-0----WildlifeMammalN11672204428116pos13i3c0-4f02l0BirdsN2380000500pos1j0----0-
a) All samples are analysed based on suspision of disease and does not reflect the country prevalence.b) 1 orangutan, 1 spider monkey, 1 barbary ape.c) 1 roe deer, 1 fox, 1 mink.d) 1 chinchilla.e) 4 ring-tailed lemurs, 1 capybara, 1 golden lion tamarin.f) 3 roe deer, 1 hedgehog.g) 1 elephant, 1 lizzard.h) 1 rainbow lorikeet.i) 12 European hedgehogs, 1 badger.j) 1 black-headed gull.k) In 2007, Denmark achieved official status as region with negligible risk ofTrichinella,according to EU Regulation (EC) No2075/2005.l) Two mink out of 142 was positive withTrichinella pseudospiralis.Source: National Veterinary Institute, Technical University of Denmark
Table A24. The BovineSpongiform Encephalopathy (BSE)surveillance programme for cattle, 2008Type of surveillanceNPositiveActive surveillancea,bHealthy slaughtered animals (>30 month)190,8240Risk categories:Emergency slaugthers (>24 month)1,6120Slaughterhouse ante-mortem inspection revealed suspicion or signs ofdisease (>24 month)30Fallen stock (>24 month)40,7200Animals from herds under restriction00Passive surveillanceAnimals suspected of having clinical BSETOTAL3233,1620
a) Samples (brain stem material) are tested using a IDEXX technique or Prionics-Check PrioStrip.b) Confirmatory testing is carried out using histopathology or immunohistochemistry. Further confirmation is performed atthe Community TSE reference laboratory.Source: Danish Veterinary and Food Administration
Annual Report on Zoonoses in Denmark 2008
41
Appendix C
Appendix C
Table A25. TheTransmissible Spongiform Encephalopathy (TSE)surveillance programme forsheep and goats, 2008Type of SurveillancePositiveNa,bActive surveillanceFallen stock (>18 mo.)8,7882Healthy slaughtered animals (>18 mo.)00Animals from herds under restriction00Passive surveillanceAnimals suspected of having clinical TSETOTAL08,78802
a) Samples (brain stem material) are tested using a IDEXX technique.b) Confirmatory testing is carried out using histopathology or immunohistochemistry. Further confirmation isperformed at the Community TSE ferefence laboratory.Source: Danish Veterinary and Food Administration
a) The genotypes were grouped in the NSP classification system accordingto their different susceptibility:NSP 1: Genetically most resistant. NSP 2: Genetically resistant.NSP 3: Genetically little resistance. NSP 4: Genetically susceptible.NSP 5: Genetically highly susceptible.Source: National Veterinary Institute, Technical University of Denmark
Table A26. Distributiona(%) of prion protein genotype of sheeprandomly selected, 2008SheepGenotypen=102NSP 1ARR/ARR29.4NSP 2ARR/AHQ2.0ARR/ARQ11.8ARR/ARH/Q2.0NSP 3 (ARQ/ARQ) ARQ/ARQ28.4NSP 3 (Other)AHQ/AHQ2.0ARH/ARH1.0ARQ/ARH2.9ARQ/AHQ8.8NSP4ARR/VRQ2.9NSP5ARQ/VRQ8.8Total100
Table A27. The Cronic Wasting Disease (CWD) surveillance programme for deer, 2008Type of SurveillancePositiveNa,bActive surveillanceWild deer - road injured/hunted (>18 mo.)450Farmed deer - culled (>18 mo.)350TOTAL800a) Samples (brain stem material) are tested using a IDEXX technique.b) Confirmatory testing is carried out using histopathology or immunohistochemistry. Further confirmation isperformed at the Community TSE reference laboratory.Source: Danish Veterinary and Food Administration
42
Annual Report on Zoonoses in Denmark 2007
Appendix C
Table A28. Centrally coordinated studies conducted in 2008Agents analysis per sampleNo. ofTitle of projectsamples (regional laboratories)150E. coli, Salmonella,viraMicrobiological classification of theproduction areas for bivalve molluscsAntimicrobial resistance in Danish andimported meat (chicken, beef and pork)Campylobacterin fresh, chilled Danishchicken meatCampylobacterin fresh chilled and frozenDanish and imported chicken meat andfrozen Danish chicken meatCampylobacterin fresh chilled importedpoultry meat (duck and turkey)Intensified control forSalmonellaandCampylobacterin fresh Danish meatIntensified control forSalmonellaandCampylobacterin fresh imported meatHygiene quality and shelf life of sliced meatproductionVTEC in minced beefSalmonelladublin in Danish dairy herdSamples of different origin related to humaneoutbreak ofSalmonellaTyphimurium U292(food stuffs, swabs, pen-faecal samples)1,0008601,800Campylobacter, Salmonella,E. coli, EnterococcusCampylobacterCampylobacter
FutherinformationResults are notpresentedResults are notpresentedAppendix C, TableA11Appendix C, TableA12Results are notpresentedAppendix C, TableA20Appendix C, TableA20Results are notpresentedChapter 4Results are notpresentedResults are notpresented
800900a1,500a1003002003,100
CampylobacterSalmonella, Campylobacter(quantitative)Salmonella, Campylobacter(quantitative)Total aertobic bacterial count andlactic acid producing bacteriaVTECSalmonellaSalmonella
a) BatchesSource: Danish Veterinary and Food Administartion and National Food Institute, Technical University of Denmark
Table A29.Listeria monocytogenesin ready-to-eat foodsa, 2008Samples analysed bySamples analysed by a quantitative methoda qualitative methodSamples with Samples between Samples withNPositivebFood categorySampling place N10 and 100 cfu/g>100 cfu/g< 10 cfuc/gRTE products ofAt processing64033233200meat originAt retail127364464022Milk and dairy0products, RTEAt processing240110110Cheese, RTEAt processing2608080Fishery products,At processing110590590At retail40240243Fruit and vegetables At processing50820792At retail1102130211Other RTE products At processing120858500At retail20747400a) Samples are collected by the Regional Veterinary and Food Control Authorities according to European Regulation (EC) No2073/2005b)Listeria monocytogenespresent in a 25 g sample of the the product.c) cfu: The number of colony forming units.Source: Danish Veterinary and Food Administration
Annual Report on Zoonoses in Denmark 2008
43
Appendix DMonitoring and surveillance programmesTable A30. Overview of notifiable and non-notifiable human diseases presentedin this report, 2008Notifiable in humansNotification routeBacteriaBrucellaspp.no-Campylobacterspp.Labb1979aChlamydophila psittaciPhysicianc1980a(Ornithosis)Listeria monocytogenesPhysician1993aLeptospiraspp.Physician1980aMycobacterium bovis/1905aPhysician (and labd)tuberculosisCoxiella burnetiino-SalmonellaLab1979aVTECPhysician and Lab2000aYersinia enterocoliticaLab1979aParasitesCryptosporidiumspp.no-Echinococcus multilocularisno-Echinococcus granulosusno-Toxoplasma gondiino-Trichinellaspp.no-VirusesTelephone andLyssa virus (Rabies)1964aphysicianPrionsTSEBSE/Creutzfeld Jacob-1997a-Physician
a) Danish order no. 277 of 14/04/2000. Cases must be notified to Statens Serum Institut.b) The regional microbiological laboratories report confirmed cases.c) The physician report individually notifiable infections.d) The laboratories voluntarily report confirmed cases.Source: Statens Serum Institute and Danish Veterinary and Food Administration
44
Annual Report on Zoonoses in Denmark 2007
Appendix D
Table A31. Overview of notifiable and non-notifiable animal diseases presented in this report,2008Notifiable in animalsEU legislationDanish legislationBacteriaCattle - DecisionOrder no 305 of 3/5 2000Brucellaspp.1920e, OBF in 1979b,2004/320/ECno cases since 1962.Never detected,Sheep and goats -Order no. 739 of 21/8 2001c2004/320/ECObmF in 1995-Pigs - DirectiveOrder no. 205 of 28/3 20092003/99/ECCampylobacterspp.no--Chlamydophila psittaciyes-Birds and poultry - order(Ornithosis)no. 78 of 30/1 1997Listeria monocytogenesno--Leptospiraspp. (only inyes-Act no. 432 of 09/06/2004production animals)Mycobacterium bovis/Cattle - DecisionCattle - Order no. 1417 of1920etuberculosis2004/320/EC11/12 2007OTF since 1980dCoxiella burnetii2005-Act no. 432 of 09/06/2004-Cattle/swine - Order no.Salmonella1993a112 of 24/02/2005VTECno--Yersinia enterocoliticano--ParasitesCryptosporidiumspp.no--Echinococcus multilocularis2004Council directiveAct no. 432 of 09/06/200464/433/ECEchinococcus granulosus1993Council directiveAct no. 432 of 09/06/200464/433/ECToxoplasma gondiino--eCircular no. 9466 ofRegulationTrichinellaspp.19202075/2005/EC12/07/2006VirusesLyssa virus (Rabies)1920-Order no. 14 of 11/01/1999and Order no. 914 of15/12/1987Order no. 930 ot07/09/2006Order no. 800 of13/07/2006
PrionsTSE
yes
Sheep & goats -Regulation 999/2001/EC(as amended)Cattle - Regulation999/2001/EC (as
BSE
yes
a) Only clinical cases notifiable.b) OBF according to Council Directive 64/432/EEC as amended by Council Directive 97/12/EC and Commision Deci-sions 93/52/EEC, 2003/467/EC and 2004/320/EC.c) ObmF according to Council Directive 91/68/EEC and Commision Decisions 93/52/EEC, 94/877/EEC, 2003/467/ECand 2004/320/EC.d) OTF according to Council Directive 64/432/EEC as amended by Council Directive 97/12/EC and regulation (EC)1226/2002, and Commission Decision 2003/467/EEC.e) Clinical cases, observations during the meat inspection at the slaughterhouse, positive blood sampes or finding ofagens are notifiable.Source: Statens Serum Institute and Danish Veterinary and Food Administration
Annual Report on Zoonoses in Denmark 2007
45
Appendix D
Appendix D
Table A32.Salmonellasurveillance programme for the rearing breeding flocks and adult breedingflocks of the grandparent and parent generation of the broiler and table egg production, 2008Parent generationRearing flocksGrandparent generationTimeSample taking MaterialMaterialPer delivery5 transport crates from one5 transport crates from oneDay-olda,bdelivery: crate liners (>1m2in total) delivery: crate liners (>1m2in total)or swab samples (>1m2in total).or swab samples (>1m2in total).Analysed as one pool.Analysed as one pool.1st& 2ndweekb, fPer unitc-2 pairs of boot swabs (analysed asone pooled sample) or 1 faecessample of 60 gram.
4thweeka,b
5 pairs of boot swaps (analysed as 2 pairs of boot swabs (analysed astwo pooled samples), or 1 faecesone pooled sample) or 1 faecessample consisting of 2x150 gram. sample of 60 gram.Per unit2 pairs of boot swabs (analysed as 2 pairs of boot swabs (analysed asone pooled sample), or 1 faecesone pooled sample) or 1 faecessample of 60 gram.sample of 60 gram.2 pairs of boot swabs (analysed asone pooled sample) or 1 faecessample of 60 gram.Parent generationMaterialHatcher basket liners from 5baskets (>1m2in total) or 10g ofbroken eggshells from each of 25hatcher baskets (reduced to 25gsub-sample). Analysed as one pool.
8thweekb,f
5 pairs of boot swabs (analysed astwo pooled samples), or 1 faecessample consisting of 2x150 gram.Production flocksGrandparent generationTimeSample taking MaterialHatcher basket liners from 5Every two weeksbPer flockbaskets (>1m2in total) or 10g of(Every 16thbroken eggshells from each of 25weekd)ehatcher baskets (reduced to 25gsub-sample). Analysed as one pool.2 weeks prior tomovinga,dAfter each hatchbPer hatchEvery weekbPer unit
Per unit
Wet dust samples. Up to 4 hatchers Wet dust samples. Up to 4 hatchersof the same flock can be pooled.of the same flock can be pooled.-2 pairs of boot swabs (analysed asone pooled sample) or 1 faecessample of 60 gram.
0-4 weeks aftermoving, 8-0weeks beforeAfter positivefindingsdd
Per unit
5 pairs of boot swabs (analysed as 5 pairs of boot swabs (analysed astwo pooled samples), or 1 faecestwo pooled samples), or 1 faecessample consisting of 2x150 gram. sample consisting of 2x150 gram.5 pairs of boot swabs (analysed as 5 pairs of boot swabs (analysed astwo pooled samples).two pooled samples).
Per unit
a) Sampling requirements set out by Regulation (EC) 2160/2003.b) Samples collected by the food business operator.c) A unit (house) may harbor part of a flock or more than one flock, depending on the size of the unit.d) Samples collected by the Regional Veterinary and Food Control Authorities.e) When eggs from a flock exceed the capacity of one incubator, each incubator should be sampled as described.f) Order no 1259 of 15/12/2008.Source: Danish Veterinary and Food Administration
46
Annual Report on Zoonoses in Denmark 2007
Appendix D
Table A33.Salmonellasurveillance programmeafor the broiler flocks, 2008Broiler productionTimeSamples taken Material15 - 21 days beforePer flock5 pairs of boot swabs. Analysed individually.slaughter, Ante mortem(AM)b,c7 - 10 days beforePer flock5 pairs of boot swabs. Analysed individually.slaughter, Ante mortem(AM)dAfter slaughterPer batchSampling is depending on whether the slaughterhousebslaughters only AM-negative flocks or AM-negative as well asPost mortem (PM)AM-positive flocks.a) According to Order no 1261 of 15/12/2008.b) Samples collected by the food business operator.c) Once a year, the samples are collected by the Regional Veterinary and Food Control Administration.d) Samples are collected by a representative of the slaughter house, laboratorium or the Regional Veterinary and Food ControlAdministration.Source: Danish Veterinary and Food Administration
Table A34.Salmonellasurveillance programme for the pullet-rearing, layer and barnyard/hobby flocks inthe table egg production, 2008Pullet-rearing flocksTimeSample taking Materiala,dPer delivery5 transport crates from one delivery: Crate liner (> 1 m2inDay-oldtotal) or swab samples (> 1 m2in total) (Analysed as onepooled sample).4 weeks oldb,d2 weeks before movinga,cPer flockPer flock5 pairs of boot swabs. Analysed as two pooled samples. Cagebirds: 5x60 samples of fresh droppings (1g).5 pairs of boot swabs. Analysed as two pooled samples. Cagebirds: 5x60 samples of fresh droppings (1g). 60 blood samples(serology).
Layers (Production for certified packing stations)TimeSample taking Materiala,cPer flock2 pairs of boot swabs (analysed as one pooled sample) or 124 weeks oldfaeces sample consisting of 2x150 gram. 250 ml (100 g) dust or1 pair of boot swabs. 60 eggsb(serology).Every 9 weeksd,ePer flock2 pairs of boot swabs. Analysed as one pooled sample. Cagebirds: 60 samples of fresh drop-pings (1g). Analysed as onepool. 60 eggsb(serology).
Barnyard and hobby flocksTimeEvery 18 weeksd
Sample taking MaterialPer folckEgg samples.
a) Sampling requirements set out by Regulation (EC) 2160/2003.b) According to Order no 1260 of 15/12/2008.c) Samples collected by the Regional Veterinary and Food Control Administration.d) Samples collected by the food business operator.e) According to Regulation (EC) 2160/2003 sample collection must be carried out every 15 weeks as a minimum.Source: Danish Veterinaty and Food Administration
Annual Report on Zoonoses in Denmark 2007
47
Appendix D
Appendix D
a) According to Order no 1261 of 15/12/2008.b) Samples collected by the food business operator.Source: Danish Veterinary and Food Administration
Table A35.Salmonellasurveillance programmesafor ducks and turkey flocks, 2008Duck productionTimeSamples takenMaterialMax. 21 days before slaughter, AntePer flock2 pairs of boot swabs. Analysedbindividually.mortem (AM)Turkey productionTimeSamples takenMaterialMax. 21 days before slaughter, AntePer flock5 pairs of boot swabs. Analysedbindividually.mortem (AM)
Table A36.SalmonellaDublin surveillance programmeafor the cattle herds andSalmonellasurveillance programme at slaughter, 2008Milk producing herdsNo. of testsSample takenHerd level1a4 samples distributed over 13 months Tank milk8 samplesNon-milk producing herdsNo. of tests1 sampleb
Blood samplesSample takenBlood samples
1bHerd level1b2 ->1b
If the owner wants a herd moved from Blood sampleslevel 2 to 1bcBeef carcasses at the slaughterhouseNo. of samples5 samples daily pooled into oneanalysis5 samples per 200 slaughtered cattlepooled into one analysisSample taken
Sampling time and no. of animalsslaughtered
Swab samples from 3 designated > 200 cattle slaughtered per dayareas after 12 hours chilling (3-100m2)Swab samples from 3 designated > 200 cattle slaughtered perareas after 12 hours chilling (3- month,≤ 200 cattle slaughtered per day100m2)
5 samples every 3rdmonth pooled into Swab samples from 3 designated > 50 cattle slaughtered per month,areas after 12 hours chilling (3- < 200 cattle slaughtered perone analysismonth100m2)1 sample every 3rdmonthSwab samples from 3 designated < 50 cattle slaughtered per monthareas after 12 hours chilling (3-100m2)
a) Order no. 112 of 24/02/2005 as ammendedb) If the herd has been tested forS.Dublin within the last 120 days or 8 samples have been tested within the last 12months no samples are taken.c) Number of samples equals total number of animals in the herd minus 2 (max. 8 animals, min. 4 animals).Source: Danish Veterinary and Food Administration
48
Annual Report on Zoonoses in Denmark 2007
Appendix D
Table A37.Salmonellasurveillance programme for the pig production, 2008Breeding- and multiplier herdsTimeSample takenPurposeEvery month10 blood samples perCalculation ofSalmonella-indexepidemiological unitbased on the mean from the lastthree months with most weight tothe result from the more recentmonths (1:3:6)Max. twice per yearSow-herdsTimeWhen purchaser of piglets isassigned to level 2 or 3, max. twiceper yearSlaughter-pig herdsTimeAt slaughterHerds withSalmonella-index 5or above: Pen-faecal samplesaSample takenPen-faecal samplesClarify distribution and type ofinfection in the herdPurposeClarify distribution and type ofinfection in the herd, and clarifypossible transmission from sowherds to slaughter-pig herdsPurposeCalculation of slaughter-pig indexbased on the mean from the lastthree months with most weight tothe result from the most recentmonth (1:1:3). Assigning herds tolevel 1-3 and assigning herds to risk-based surveillance (RBOV)bClarify distribution and type ofinfection in the herdTime and no. of animals slaughtered> 200 pigs slaughtered per day
Sample takenMeat juice, 60-100 samples perherd per year.Herds in RBOVa: one meat juicesample per month
Herds assigned to level 2 or 3,max. twice per year
Pen-faecal samplesc
Pork carcasses at the slaughterhouseNo. of samplesSample taken5 samples daily, pooled into oneSwab samples from 3 designatedanalysisareas (3x100 cm2) after min. 12 hchilling5 samples per 200 slaughtered pig,pooled into one analysis5 samples every 3rdmonth, pooledinto one analysis1 sample every 3rdmonthSwab samples from 3 designatedareas (3x100 cm2) after min. 12 hchillingSwab samples from 3 designatedareas (3x100 cm2) after min. 12 hchillingSwab samples from 3 designatedareas (3x100 cm2) after min. 12 hchilling
> 200 pigs slaughtered per month,≤ 200 pigs slaughtered per day> 50 pigs slaughtered per month,< 200 pigs slaughtered per month< 50 pigs slaughtered per month
a) The herd owner must inform buyers of breeding animals about the infection level and type ofSalmonella.b) RBOV: risk-based surveillance where the sample size in herds with a SP-index of zero (no positive samples in the pre-vious three months) are reduced to one sample per month.c) Producers are paid a reduced price per animal. Pigs from herds in Level 3 must be slaughtered under special hygienicprecautions.Source: Danish Veterinary and Food Administration
Annual Report on Zoonoses in Denmark 2007
49
Appendix EPopulation and slaughter dataHuman population, 2008Age group (years)0-45-1415-2425-4445-64> 65TotalSource: Statistics Denmark
males166,580350,693326,145758,646738,401372,2012,712,666
females159,026333,618311,915744,561733,165480,8402,763,125
Total325,606684,311638,0601,503,2071,471,566853,0415,475,791
a) Animals are slaughtered abroad.Source: The Central Husbandry Register, Statistics Denmark and Danish Veterinary andFood Administration
Number of herds/flocks, livestock and animals slaughtered, Dec 2008LivestockNumberHerds/flocks(capacity)slaughteredSlaughterpigs11,00012,195,00018,582,28832,0001,600,000Cattle511,300580Broilers20,000,000100,304,000Layers (excl. barnyard)2152,900,000-a51Turkeys482,000-a9,080Sheep & lambs173,00089,5203,475Goats23,0002,140Horses--2,520
Number of farms in the broiler production, 2008No. of holdingsRearing period (grand parent)Production period (grand parenRearing period (parent)Production period (parent)HatcheriesBroilers2416465243No. ofhouses/flocks51892154-580Livestock(capacity)50,00050,000130,000720,000-n.a.a
a) Not available.Source: Danish Veterinary and Food Administration and Danish Agriculture and FoodCouncil
50
Annual Report on Zoonoses in Denmark 2008
Appendix E
Number of farms in the table egg production, 2008No. of holdingsRearing period (parent)Production period (parent)HatcheriesPullet-rearingLayers (excl. Barnyard)35593215No. ofhouses/flocks45155295Livestock(capacity21,00033,0001,420,0002,900,000
Source: Danish Veterinary and Food Administration and Danish Agriculture and FoodCouncil
Distribution of import, export and production of fresh and frozen meat andthe production of table eggs in Denmark, 2007-2008. Data is presented intonsBroiler Turkey Duck TablePorkaBeefameatbmeatcmeatdeggseImport200740,20180,28730,3908,4233,845-200883,05781,42732,4808,2644,494-ExportProductionConsumptionf2007200820072008200720081,263,1691,386,8491,447,8941,602,648224,925298,85761,37466,690134,374149,744153,287164,481105,741109,725168,354157,54393,00380,2981,6922,34534496,7655,9684547722,956376,3473,722--66,800g67,900g--
a) Salted, smoked, dried, cooked or preserved meat is not included.b) Cooked or preserved meat is not included. Natural-marinated chicken is included (pro-duct line 1602 3211).c) Cooked or preserved meat is not included.d) Salted, smoked or dried meat or mixed products of ducks, geese and guinea fowl are notincluded.e) Include fresh table eggs from battery, free-range, organic and deep litter production andtable eggs for producers´ own use and barnyard sale.f) Consumption = Production + import - exportg) Consumption of table eggs is assumed to be roughly the same as the production, sinceimport and export of table eggs is minimal.Source: Statistics Denmark
Annual Report on Zoonoses in Denmark 2007
51
Appendix FList of FiguresFigure 1.1. Total incidence of human salmonellosis and estimated human incidence due to broilers, pork, table eggsand imported foods in Denmark, 1988 to 2008Figure 1.2. Estimated sources of 3,656 cases of human salmonellosis in Denmark, 2008Figure 1.3. Sources of antimicrobial resistantS.Typhimurium infections in humans, 2004-2008Figure 2.1. Aetiology of foodborne disease outbreaks reported with a causative agent in the Food- and waterborneOutbreak Database (FUD), 2008Figure 4.1. VTEC O157-prevalence in cattle, 1999-2008Figure 6.1. Overview of the monitoring and outbreak investigation network for reporting infectious pathogens inhumans, animals, foodstuffs and feedstuffs in DenmarkFigure A1. Geographical distribution of human cases per county and incidence of human infections withS.Enteriti-dis, 2008Figure A2. Geographical distribution of human cases per county and incidence of human infections withS.Typhi-murium, 2008Figure A3. Geographical distribution of human cases per county and incidence of human infections withCampylo-bacter,2008Figure A4. Geographical distribution of human cases per county and incidence of human infections withYersinia,2008Figure A5. Geographical distribution of human cases per county and incidence of human infections with VTEC,2008Figure A6. Serological surveillance ofSalmonellain breeding and multiplying pigs based on monthly testing of bloodsamples, 2003-2008Figure A7. Serological surveillance ofSalmonellain slaughterpigs, 2003-2008Figure A8.Salmonellain pork, monitored at slaughterhouses, 2003-2008Figure A9.Salmonellain beef, monitored at slaughterhouses, 2003-2008
52
Annual Report on Zoonoses in Denmark 2008
Appendix F
List of TablesTable 3.1. Methods used in the surveillance of foodborne pathogens in DenmarkTable A1. Estimated no. of reported human cases and percentage of cases per major food source, travel or outbreaks,2006-2008Table A2. Zoonoses in humans, number of laboratory-confirmed cases, 1999-2008Table A3. VTEC O-group distribution in humans, 2008Table A4. Foodborne disease outbreaks reported in the Food- and waterborne Outbreak Database (FUD), 2008Table A5. Serotype distribution (%) ofSalmonellafrom humans, animals, carcasses at slaughterhouse and importedmeat, 2008Table A6. Phage type distribution (%) ofS.Enteritidis from humans, animals and imported meat, 2008Table A7. Phage type distribution (%) ofS.Typhimurium from humans, animals and imported meat, 2008Table A8. Occurrence ofSalmonellain the table egg production, 2000-2008Table A9. Occurrence ofSalmonellain the table egg layer flocks according to type of production, 2000-2008Table A10. Occurrence ofSalmonellain the broiler production, 2000-2008Table A11. Occurrence ofCampylobacterin broiler flocks and in fresh meat at slaughter, 2000-2008Table A12. Occurrence ofCampylobacterin non-heat treated broiler meat at retail, 2001-2008Table A13. Relative distribution ofCampylobacterspecies (%) in broilers before slaughter, 2002-2008Table A14. Occurrence ofSalmonellain turkey and duck flocks, 2006-2008Table A15. Occurrence of zoonotic pathogens in pigs and pork in Denmark, 2008Table A16. Occurrence of zoonotic pathogens in cattle and beef in Denmark, 2008Table A17. Cattle herds assigned to level 1-3 according to theS.Dublin surveillance programme, January 2009Table A18. Isolation ofSalmonellafrom outbreaks of clinical disease in pig and cattle herds, 2008Table A19. Distribution ofCampylobacter(%) in pig and cattle herds, 2000-2008Table A20. Results from the intensified control ofSalmonellaandCampylobacterin fresh meat based on a case-by-case risk assessment, 2008Table A21. Control ofSalmonellain compound feeds, feed processing and feed material, 2004-2008Table A22. Three categories of meat and bone meal by-products not intended for human consumption, 2008Table A23. Occurrence of zoonotic pathogens in pets, zoo animals and wild life in Denmark, 2008Table A24. The BovineSpongiform Encephalopathy(BSE) surveillance programme for cattle, 2008Table A25. The TransmissibleSpongiform Encephalopathy(TSE) surveillance programme for sheep and goats, 2008Table A26. Distribution (%) of prion protein genotype of sheep randomly selected, 2008Table A27. The Cronic Wasting Disease (CWD) surveillance programme for deer, 2008Table A28. Centrally coordinated studies conducted in 2008Table A29.Listeria monocytogenesin ready-to-eat foods, 2008Table A30. Overview of notifiable and non-notifiable human diseases presented in this report, 2008Table A31. Overview of notifiable and non-notifiable animal diseases presented in this report, 2008Table A32.Salmonellasurveillance programme for the rearing breeding flocks and adult breeding flocks of the grand-parent and parent generation of the broiler and table egg production, 2008Table A33.Salmonellasurveillance programme for the broiler flocks, 2008Table A34.Salmonellasurveillance programme for the pullet-rearing, layer and barnyard/hobby flocks in the tableegg production, 2008Table A35.Salmonellasurveillance programmes for ducks and turkey flocks, 2008Table A36.SalmonellaDublin surveillance programme for the cattle herds andSalmonellasurveillance programme atslaughter, 2008Table A37.Salmonellasurveillance programme for the pig production, 2008
Annual Report on Zoonoses in Denmark 2008
53
Contributing Institutions:Danish Zoonosis CentreThe National Food InstituteTechnical University of DenmarkMørkhøj Bygade 19DK - 2860 SøborgTel: +45 3588 7000E-mail: [email protected]www.food.dtu.dkStatens Serum InstitutArtillerivej 5DK - 2300 København STel: +45 3268 3268E-mail: [email protected]www.ssi.dkThe Danish Veterinary and Food AdministrationThe Regional Veterinary and Food Control AuthoritiesMørkhøj Bygade 19DK - 2860 SøborgTel: +45 3395 6000E-mail: [email protected]www.fvst.dkThe National Veterinary InstituteTechnical University of DenmarkBülowsvej 27DK - 1790 Copenhagen VTel: +45 3588 6000E-mail: [email protected]www.vet.dtu.dkThe Danish Plant DirectorateSkovbrynet 20DK - 2800 LyngbyTel: +45 4526 3600E-mail: [email protected]www.pdir.dkDanish Agriculture and Food CouncilAxelborg, Axeltorv 3DK - 1609 Copenhagen VTel: +45 3339 4000E-mail: [email protected]www.lf.dk
54
Annual Report on Zoonoses in Denmark 2008
National Food InstituteTechnical University of DenmarkMørkhøj Bygade 19DK - 2860 SøborgT: 35 88 70 00F: 35 88 70 01www.food.dtu.dkISSN: 1600-3837