Udvalget for Fødevarer, Landbrug og Fiskeri 2008-09
FLF Alm.del Bilag 267
Offentligt
Journal of Gerontology: MEDICAL SCIENCES2000, Vol. 55A, No. 10, M585–M592
Copyright 2000 by The Gerontological Society of America
Worldwide Incidence of Hip Fracture in Elderly Women:Relation to Consumption of Animal andVegetable FoodsLynda A. Frassetto, Karen M. Todd, R. Curtis Morris, Jr., and Anthony SebastianDepartment of Medicine and General Clinical Research Center, University of California, San Francisco.
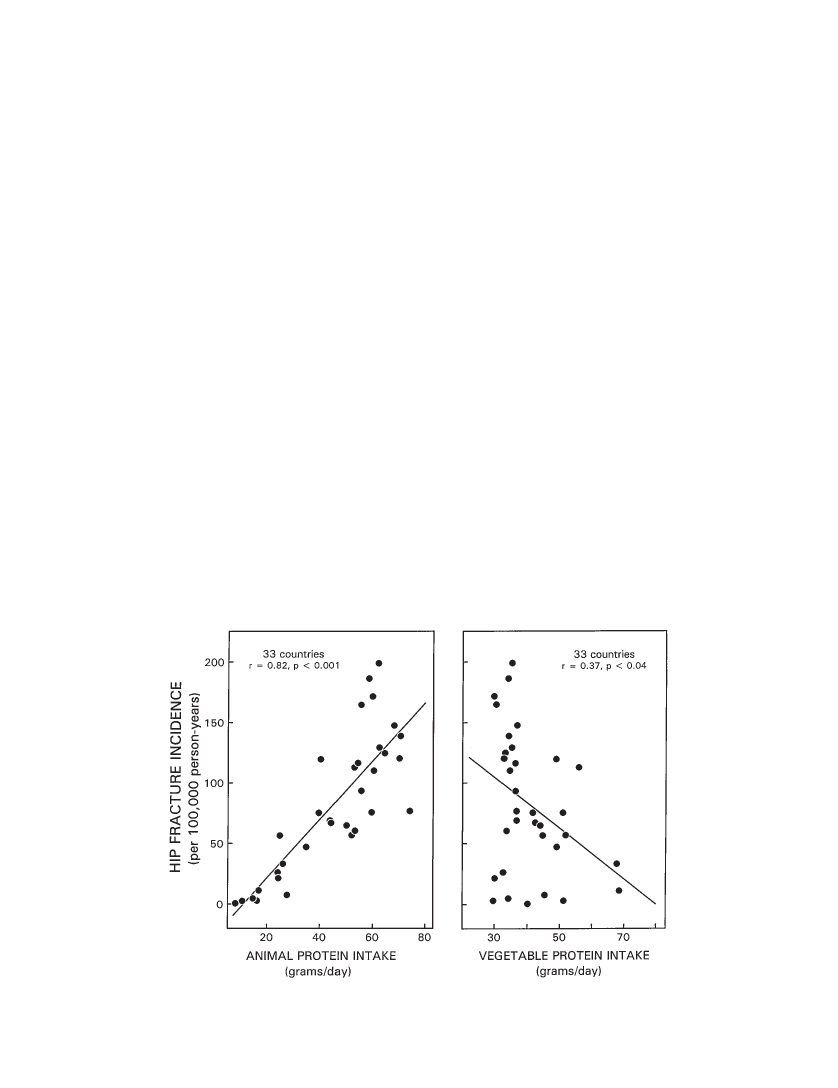
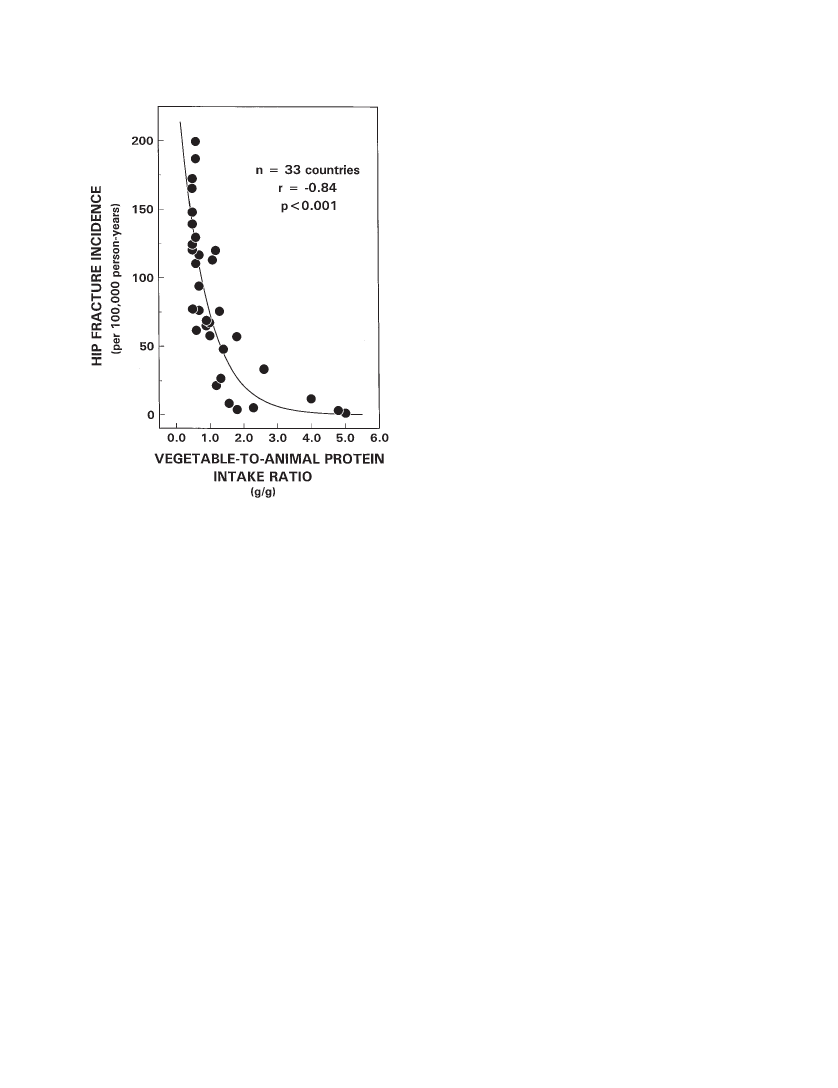
Background.Hip fracture, a major health problem in elderly persons, varies in incidence among the populations ofdifferent countries and is directly related to animal protein intake, a finding that suggests that bone integrity is compro-mised by endogenous acid production consequent to the metabolism of animal proteins. If that is so, vegetable foodsmight provide a countervailing effect, because they are a rich source of base (bicarbonate) in the form of metabolizableorganic anions, which can neutralize protein-derived acid and supply substrate (carbonate) for bone formation.Methods.We analyzed reported hip fracture incidence (HFI) data among countries (N33) in women aged 50years and older, in relation to corresponding country-specific data on per capita consumption of vegetable and animalfoods as reported by the United Nations Food and Agriculture Organization.Results.HFI varied directly with total (r.67,p.001) and animal (r.82,p.001) protein intake and in-versely with vegetable protein intake (r.37,p.04). The countries in the lowest tertile of HFI (n 11) had thelowest animal protein consumption, and invariably, vegetable protein (VP) consumption exceeded the country’s corre-sponding intake of animal protein (AP): VP/AP 1.0. By contrast, among the countries in the highest tertile of HFI, an-imal protein intake exceeded vegetable protein intake in nearly every case (10 of 11 countries). Among all countries,HFI correlated inversely and exponentially with the ratio of vegetable/animal protein intake (r.84,p.001) andaccounted for 70% of the total variation in HFI. Adjusted for total protein intake, vegetable food consumption was an in-dependent negative predictor of HFI. All findings were similar for the subset of 23 countries whose populations are pre-dominantly Caucasian.Conclusion.The findings suggest that the critical determinant of hip fracture risk in relation to the acid-base effectsof diet is the net load of acid in the diet, when the intake of both acid and base precursors is considered. Moderation ofanimal food consumption and an increased ratio of vegetable/animal food consumption may confer a protective effect.
I
N 1992, Abelow and coworkers reported that the inci-dence of hip fractures in women aged 50 years and oldercorrelates positively with a country’s average annual percapita consumption of animal protein (1). Noting that ani-mal protein is a rich source of sulfur-containing aminoacids, which the body metabolizes to the fixed acid sulfuricacid, Abelow and coworkers interpreted the positive corre-lation of hip fracture rate and animal protein intake as con-sistent with the hypothesis that hip fractures result in partfrom deleterious effects of prolonged exposure to dietaryacid (2,3).If that interpretation is correct, however, the decisive riskfactor for hip fracture would not be the rate of production offixed acid (sulfuric acid) from animal protein but the netrate of endogenous acid production, when all sources of di-etary acid and base are considered. Net endogenous acidproduction is determined by dietary factors in addition toanimal protein intake, including factors that result in endog-enous base production and that attenuate or counteract theacid-producing effect of animal protein (4). Vegetable foodsin particular are rich in bicarbonate and in organic anionsthat can be metabolized to bicarbonate (5,6), which in turnreduces the net rate of endogenous acid production for agiven rate of sulfuric acid production (7).
Accordingly, for any protein intake, the net rate of endog-enous acid production may vary over a substantial range,depending on the relative intakes of animal and vegetablefoods. Taking vegetable food consumption into consider-ation, therefore, is necessary to fully assess the net acid-base effect of diet and to determine whether the hip fracturerate correlates with net endogenous acid production. Onecannot predict a priori whether the contribution of vegetablefoods will weaken or strengthen the case for a positive asso-ciation between hip fracture rate and net acid productionbased on consideration of animal foods consumption alone.This study examines the cross-cultural relation betweenhip fracture incidence (HFI) and acid-base potential of thediet, taking into consideration the consumption of both veg-etable and animal foods.METHODS
Country-specific surveys of HFI were identified by Med-line searches and pursuit of the generated leads. Reportswere selected only if hip fracture rates were recorded forwomen over the age of 50 years and if per capita food con-sumption data were available for the country surveyed (seebelow). Eighty-seven surveys from 33 countries met thesecriteria (8–74).M585
M586
FRASSETTO ET AL.
For each survey, hip fracture rates for women over theage of 50 were expressed as fractures per 100,000 person-years for each age group tabulated in the report. Age group-ing varied among reports, most often in 5- or 10-year inter-vals. To allow between-country comparison of fracturerates, the fracture rate for each age group in each survey wasadjusted to a common population base for that age group,using the direct method of standardization (75). The refer-ence population for that standardization was the age groupdistribution of women in the United States for 1987, as re-ported by the US Census Bureau (76). For each survey, thecumulative standardized fracture rate for all age groups over50 years was then calculated. For each of the 33 countriessurveyed, a single estimate of fracture incidence was gener-ated as the mean of the standardized cumulative fracturerates from all surveys for that country, and that value wasused to represent hip fracture incidence for between-countrycomparisons and relation to food consumption parameters.Estimates of per capita consumption of foods of both ani-mal and vegetable origin were obtained fromFood BalanceSheets(77), published by the Food and Agriculture Organi-zation (FAO) of the United Nations. Whenever possible, for
each hip fracture survey, the food consumption data usedwas that for the 10-year interval prior to the date of the frac-ture survey, averaged over the interval. Otherwise, data forthe closest available prior decade were used. For each coun-try, a single estimate of each per capita food consumptionvariable (see below) was then generated as the mean of thevalues for all surveys. The food consumption variables eval-uated were animal protein (AP), vegetable protein (VP), to-tal protein (TP), in grams/day, their ratios, and the quantitiesof animal, vegetable, and total foods consumed, in kilocalo-ries/day, and their ratios.Age-adjusted hip fracture rates were analyzed in relationto food consumption variables by linear, multiple linear,and nonlinear regression, using SigmaStat (Jandel Corp.,San Raphael, CA).RESULTS
Table 1 lists the averages by country (N33) of HFI ar-ranged in increasing order and the corresponding values ofper capita consumption (g/day) of animal, vegetable, and to-tal protein. Hip fracture incidence varied greatly, from lessthan 1.0 (Nigeria) to nearly 200 (Germany) per 100,000 per-
Table 1. Hip Fracture Incidence (HFI) and Dietary Protein Intake by CountryHFI per100,000Person-Years0.82.93.15.07.711.521.626.633.547.356.857.260.765.167.369.275.576.077.093.5110.3113.0116.5119.8120.3124.8129.4139.0147.8165.1172.0186.7199.319.7 18.476.8 18.1147.3 27.8AnimalProtein Intake(AP) (g/day)8.110.716.314.727.816.924.524.326.135.025.052.153.350.144.344.039.759.674.255.760.453.154.440.770.164.762.670.668.255.659.958.662.420.9 7.753.3 9.060.7 8.2VegetableProtein Intake(VP) (g/day)40.251.229.734.345.468.630.232.767.849.144.851.933.644.142.536.751.041.736.736.434.755.936.348.932.933.335.234.336.930.529.834.035.344.9 13.142.3 7.335.2 4.8Total Protein(TP) (g/day)48.361.946.049.073.285.554.757.093.984.069.8104.086.994.286.880.790.7101.3110.992.195.1109.190.789.5103.198.097.8104.9105.086.189.792.597.765.8 15.995.6 9.295.9 6.3
Tertile of HFILowest
CountryNigeriaChinaNew GuineaThailandSouth AfricaKoreaSingaporeMalaysiaYugoslaviaSaudi ArabiaChileItalyHollandSpainJapanHong KongIsraelIrelandFranceFinlandCanadaCreteUnited KingdomPortugalUnited StatesAustraliaSwitzerlandNew ZealandArgentinaDenmarkSwedenNorwayGermanymeanSDmeanSDmeanSD
VP/AP5.04.81.82.31.64.01.21.32.61.41.81.00.60.91.00.91.30.70.50.70.61.10.71.20.50.50.60.50.50.50.50.60.62.5 1.30.8 0.20.6 0.2
Middle
Highest
Lowest tertileMiddle tertileHighest tertile
DIET ACID LOAD AND HIP FRACTURE INCIDENCE
M587
son-years. Among the countries in the lowest tertile of HFI(n11 countries), hip fracture rates were less than 60 per100,000 person-years. These 11 countries also had the low-est animal protein consumption of the 33 countries sur-veyed. Notably, in all 11 countries in the lowest tertile ofHFI, vegetable protein consumption exceeded the country’scorresponding intake of animal protein (VP/AP 1.0). Bycontrast, among the countries in the highest tertile of HFI( 116 per 100,000 person-years), animal protein intake ex-ceeded vegetable protein intake in 10 of 11 countries.Among the 33 countries, HFI varied directly with totalprotein intake (HFI97.92.1 ¶ TP,r.67,R2.45,p.001), directly with animal protein intake (HFI26.591 2.413 ¶ AP,r.82,R2.67,p.001) (Fig-ure 1, left panel), and inversely with vegetable protein intake(HFI 167.272 2.093 ¶ VP,r.37,R2.14,p.04)(Figure 1, right panel). Hip fracture incidence was stronglycorrelated inversely and exponentially with the ratio of vege-table/animal protein intake (HFI 257.6 ¶e( 1.24 ¶ VP/AP),R2.84,p.001) (Figure 2). Thus, 70% ( 0.842¶ 100) ofthe variation in HFI among countries was accounted for bythe variation in vegetable/animal protein ratio. Amongcountries, vegetable and animal protein intakes correlatedinversely (AP0.19 ¶ VP 49.5,r.37,R2.14,p.04).Adjusting for animal protein intake in multiple regressionanalysis, vegetable protein intake was not a significant pre-dictor of HFI. Animal protein intake, however, correlateddirectly with total protein intake (AP –33.5 0.91 ¶ TP,r.86,p.001), whereas vegetable protein intake didnot correlate significantly with total protein intake. Afteradjusting for total protein intake in multiple regression anal-
ysis, vegetable protein intake was a significant negative pre-dictor of HFI (HFI2.3 ¶ TP 2.8 ¶ VP 5.9,R2.68,p.001).Reanalysis of the data expressing per capita consumptionof animal and vegetable foods in kilocalories per day in-stead of in grams of protein per day yielded essentially iden-tical results. For example, HFI varied inversely and expo-nentially with the ratio of vegetable/animal kilocalories perday (VK/AK) consumed (HFI 212.5 ¶e0.32 (VK/AK),R.82,p.001).DISCUSSION
The findings in this study confirm those of Abelow andcoworkers (1) that HFI in women over the age of 50 is di-rectly correlated with animal protein consumption. By ex-tending the findings from 16 to 33 countries, spanning sixcontinents, the present findings greatly strengthen the gen-eralization of a worldwide association of hip fractures inwomen with animal protein consumption. The findings fur-ther extend the observations of Abelow and coworkers bydemonstrating that HFI also correlates with vegetable pro-tein consumption, but in the opposite direction.The association of hip fractures with animal protein con-sumption was interpreted by Abelow and coworkers (1) assupporting the hypothesis that eating animal foods increaseship fracture risk by increasing endogenous fixed acid pro-duction from metabolism of the accompanying protein, andthereby promotes the development of osteoporosis (2). Wereasoned that if this interpretation is correct, the critical riskfactor for hip fracture would not be the rate of production offixed acid (e.g., sulfuric acid) from animal protein but thenet rate of endogenous acid production, when sources of di-
Figure 1. Cross-cultural relationship (N 33 countries) between hip fracture incidence in women aged 50 years and older (per 100,000 per-son-years) and per capita animal protein (left panel) and vegetable protein consumption (right panel) (g/day).
M588
FRASSETTO ET AL.
Figure 2. Cross-cultural relationship (N33 countries) betweenhip fracture incidence in women aged 50 years and older (per 100,000person-years) and the ratio of vegetable-to-animal protein.
etary base as well as acid are considered. Vegetable foodsare rich in organic anions (5) that can be metabolized to thebase, bicarbonate, which in turn reduces the net rate of en-dogenous acid production for a given rate of acid produc-tion from animal foods (7,78,79). Vegetable protein intakemay therefore be a marker of the total amount of vegetablefoods ingested, which in turn indicates the amounts of base-producing organic anions ingested. (Because protein con-tent varies among vegetable foods, per unit weight or kilo-calorie of food, it is not a perfect index of the quantity ofvegetable food consumed or of the quantity of whateverconstituents are present in vegetable foods that may confer askeletal protective effect.)It seems unlikely that any beneficial effect of vegetablefood consumption on hip fracture risk would be mediatedby the proteins in the vegetable foods. Although vegetableproteins reportedly contain lesser amounts of sulfur-con-taining amino acids than do animal proteins (80), a corre-sponding lesser increase in the rate of metabolic acid pro-duction per unit increase in vegetable versus animal proteinconsumed would not be expected by itself to reverse a di-rectional effect of protein intake on hip fracture risk.[The general belief that the sulfur-containing amino acidcontent of animal proteins is greater than that of vegetableproteins is based on analyses of relatively few isolated pro-teins, whereas the more relevant issue is the relative con-tents of those amino acids in the entire complex of proteinsmaking up individual foods of animal and vegetable origin.
When the latter is examined from food composition data,the sulfur-containing amino acid content of common foodsof animal origin, expressed in mEq of sulfur per 100 g ofprotein, is fairly uniform and generally higher than mostcommon foods of vegetable origin (Table 2). But in anumber of common vegetable foods, the sulfur-containingamino acid content is within the range and greater than thatof common animal foods (Table 2). When we look at thepotential sulfuric acid load from metabolism of vegetableproteins, we must take into consideration the specific foodsingested as well as their quantity.]To further examine the influence of the relative amountsof vegetable and animal food intake on HFI, we examinedthe ratio of vegetable/animal protein intake across tertiles ofHFI. Among the countries in the lowest tertile of HFI, vege-table protein intake invariably exceeded that of animal pro-tein intake (VP/AP1), and among those in the highesttertile, vegetable protein intake was nearly always lowerthan animal protein intake (VP/AP 1; 10/11 countries)(Table 1). On average, for every hip fracture occurringamong women over the age of 50 in countries among thelowest tertile, 7 to 8 hip fractures occur among women incountries in the highest tertile. At the extremes, that is, com-paring the countries with the highest (Germany) and lowest(Nigeria) hip fracture rates, the relative fracture incidencesare 200 to 1 (Table 1). When all countries are considered to-gether, the ratio of vegetable/animal protein intake corre-lated inversely and exponentially with HFI (R.84,p.001) (Figure 2), and accounted for 70% of the total varia-tion in HFI.Because vegetable food consumption was significantlycorrelated with animal food consumption—inversely—itmight be argued that vegetable food consumption is not anindependent predictor of HFI. That is, the lower rates of hipfracture in countries in which people ate more vegetablefoods might have occurred because they also were eatingless animal foods. Yet, when total protein intake was heldconstant in multiple linear regression analysis, vegetablefood consumption remained a significant negative predictorof hip fracture rate (p.001). Thus, among countries withsimilar total protein intakes, vegetable food consumptionvaried independently and was an independent negative pre-dictor of hip fracture rate.Although HFI was more tightly correlated with animalprotein intake (r.82) than with vegetable protein intake(r.37), the magnitude of effect on hip fracture rate perunit difference in intake was nearly the same, although inopposite directions (Figure 1). In both cases, a 10-g differ-ence in protein intake was associated with a change of ap-proximately 20 hip fractures per 100,000 person-years.Consistent with the proposal of Abelow and coworkers(1) that the direct effect of animal food consumption on HFIis related to increased endogenous acid production, we pro-pose that the inverse effect of vegetable food consumptionon hip fracture is related to increased endogenous base pro-duction. The base precursors of natural foods are largely or-ganic anions, such as citrate, succinate, and other conjugatebases of carboxylic acids, which the body metabolizes to bi-carbonate. Foods contain both organic anions (e.g., citrate)and their corresponding undissociated organic acids (e.g.,
DIET ACID LOAD AND HIP FRACTURE INCIDENCE
M589
Table 2. Sulfur-Containing Amino Acid Content of CommonFoods of Vegetable and Animal Origin Expressed as PotentialAcid From Methionine and Cystine†Potential Acid FromMethionine and Cystine(mEq/100 g protein)71.166.565.458.458.158.157.557.556.756.254.353.050.446.846.3Potential Acid FromMethionine Cystine(mEq/100 g protein)46.246.244.942.541.840.439.939.138.338.336.935.232.626.926.5
Food ItemGrapesWalnutsRiceChickenWheat breadSweet cornSalmonCodHamTurkeyLambBeefBrown ricePeachesPineapple†Calculated
Food ItemOrangesBuckwheatSoybeansPotatoesBananasApplesChickpeasYamCauliflowerBlack beansZucchiniKaleTomatoesCabbageBroccoli
as 2 ¶ (methionine 2 ¶ cystine), where methionine and cystinecontents are expressed in mmol/100 g protein.
citric acid). When an ingested organic acid, such as citricacid, is metabolized by the body, the end-products are car-bon dioxide and water. When an ingested organic anion,such as citrate anion, is metabolized, the end-product is bi-carbonate (78). Vegetarian diets yield significantly lowerrates of net endogenous acid production than do mixedanimal and vegetable diets, even when the diets are equalin protein content (81). Diets that consist predominantlyof vegetable foods frequently yield negative rates of netendogenous acid production or net base production (79).Renal net acid excretion correlates positively with animalprotein intake and negatively with vegetable protein in-take (7).Careful studies indicate that diet-dependent differences innet endogenous acid production are sufficiently large to per-turb systemic acid–base equilibrium (82,83), which pre-sumably is prerequisite to initiation of pathophysiologicalsequelae. Otherwise healthy subjects eating net acid-pro-ducing diets are in a chronic state of low-grade metabolicacidosis, which increases in severity because renal functionnormally declines with increasing age (82,83). These differ-ences in systemic acid–base equilibrium induced by differ-ences both in diet net acid load and age-related renal func-tional status thus provide a potential signal for adaptiveresponses of the body that have numerous maladaptive“trade-off” effects, including dissolution of bone (84,85).Bone is a large reservoir of base, in the form of alkalinesalts of calcium that are released into the systemic circula-tion in response to increased systemic acid loads (86–89).Experimentally induced chronic metabolic acidosis by acidloading induces loss of bone mass (86,90–94) because ofparticipation of bone in this homeostatic response (86). Re-leased bone base mitigates the severity of the metabolic aci-dosis. As acid loading continues, the bone minerals accom-panying that base are wasted in the urine and bone mineralcontent, and bone mass progressively declines (91–93). The
mechanism of bone loss includes acidosis stimulation of os-teoclasts and inhibition of osteoblasts (95).Evidently, even the low-level acid loading and metabolicacidosis that occur in humans eating ordinary diets is suffi-cient to impose a chronic demand for base of skeletal origin.In humans eating ordinary net acid-producing diets, the kid-neys do not dispose of the entire daily acid load (82,96). Asa result, normal subjects are in a state of chronic acid reten-tion (82). Because the degree of acidosis in the systemic cir-culation does not increase measurably from day to day(82,86,88,96), there must be an internal reservoir supplyingbase to the systemic circulation. Bone is the only base reser-voir with sufficient capacity for that mechanism to operateover a lifetime (97). Over decades, the magnitude of dailypositive acid balance may be sufficient to induce osteoporo-sis (2,3). Reducing the diet net acid load to nearly zero bysupplementing the diet with base improves calcium balance,reduces bone resorption, and stimulates bone formation(98).Even if the findings in this study could be interpreted asindicating a causal relationship between diet net acid loadand HFI, which they cannot because they are only associa-tional, they could not be interpreted as implying that the dietnet acid load is a major determinant of hip fracture rates inelderly women. These findings do, however, raise the possi-bility only that differences in net acid load might accountfor a major fraction of the differences in fracture rate in thatsegment of the population, whatever may be setting the un-derlying acid load–independent rate. Conceivably, the ob-served magnitude of the effect of net acid load differences isdependent in part on the presence or absence of other clini-cally more important determinants of the rate of bone turn-over in elderly women, such as estrogen status, calcium in-take, body weight, and levels of physical activity.Furthermore, because differences in acid load–indepen-dent fracture risk factors perforce could not be controlledfor in this study, the possibility that the dietary net acid loadis a pathophysiologically benign covariate of one or more ofthose factors cannot be excluded. Such factors include phys-ical activity, dietary variables other than protein intake, andother physiological factors influenced by cultural practicesand state of economic development. Indeed, the issuewhether “excess” dietary protein intake adversely affectsbone in humans is a subject of current controversy in nutri-tion (3,99,100), with one group concluding that “excess pro-tein will not harm the skeleton if the calcium intake is ade-quate” (100), and another group that “excessive dietaryprotein from foods with high potential renal acid load (e.g.,animal foods) adversely affects bone, unless buffered by theconsumption of alkali-rich foods (e.g., vegetable foods)”(3). Clearly, the present study cannot be interpreted as re-solving this issue. Only the fact that the findings in thisstudy are predictable a priori from our knowledge of acid–base effects on bone make them potentially clinically andepidemiologically relevant.Nevertheless, the findings are only associational, and ofthe large numbers of potential confounders, the availabledata permitted adjusting only for age and for consideringwomen only. Heaney, in particular, has been critical of in-terpreting cross-sectional associational studies, such as this
M590
FRASSETTO ET AL.
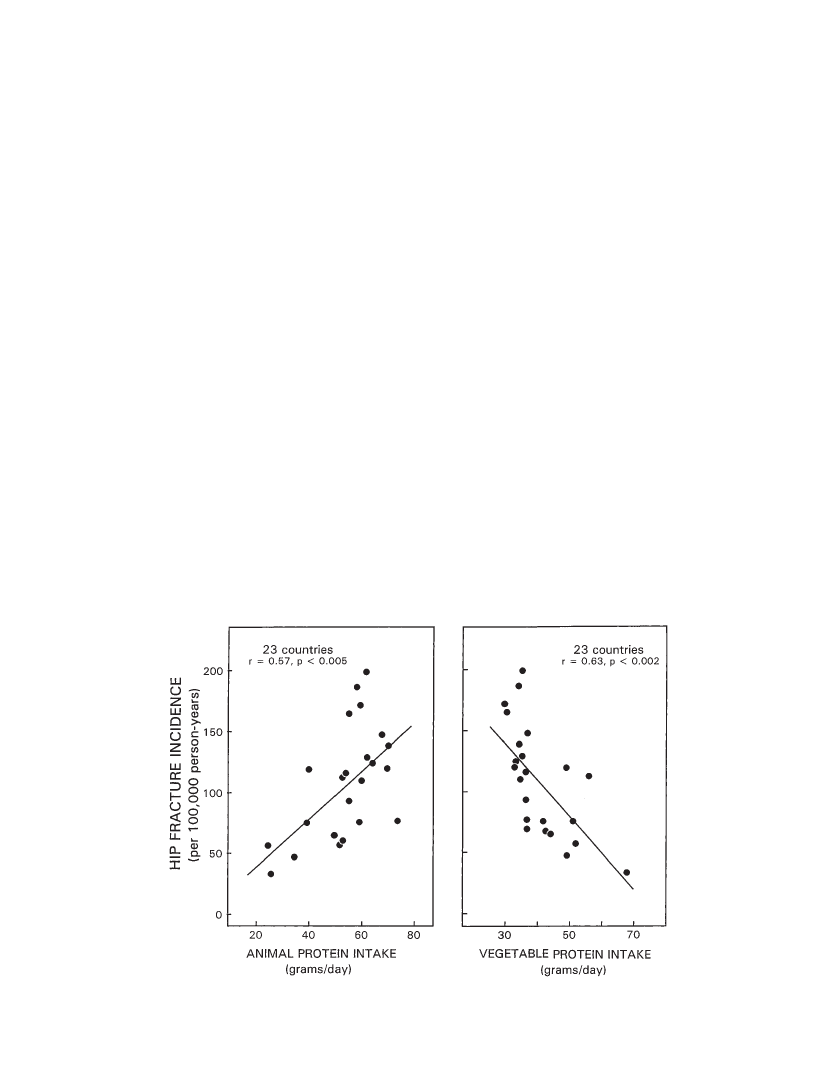
one, as suggesting causal relationship, and we share thatview. In reviewing the smaller study (n 16 countries) byAbelow and coworkers, Heaney noted, in particular, thatmany of the countries with lower hip fracture rates werepredominantly inhabited by black and Asian populations,who may have lower risks for hip fracture, either becausebone mass tends to be greater (blacks), or because the archi-tecture of the hip differs (Asian) (100). The larger numberof countries (N 33) included in the present analysis gaveus the opportunity to test whether the association of hipfracture rate and animal and vegetable food consumptioncontinues to hold when those countries (n10) are ex-cluded from the analysis. For the countries with predomi-nantly Caucasian populations (n23), we found that theresults were similar to those obtained in the primary analy-sis of all countries (Figure 3). That result does not, however,exonerate other confounders that might influence hip frac-ture risk among Caucasian women.Another potential pitfall of this particular associationalstudy stems from interpreting food consumption data (FAOFood Balance Sheets)for the combined population of acountry as applicable to one segment of the population, inthis case, women over the age of 50 (101). To the extent thatanimal and vegetable food consumption by such elderlywomen differs from the population average, the data wouldbe skewed.Based on our findings and the considerations and caveatsoffered in this discussion, we hypothesize that the relativelyhigh incidence of hip fractures in women in industrializedcountries is caused, at least in part, by the cumulative ef-fects on bone of the body’s chronic retention of a fraction ofthe high dietary net acid load characteristic of the inhabit-
ants of those countries. This high dietary net acid load, inturn, is the result of disproportionate consumption of animal(acid precursors) relative to vegetable (base precursors)foods. The degree of acid retention is determined in part bythe magnitude of the diet-determined net acid load and inpart by the acid–base regulatory integrity of the kidney,which declines with increasing age, resulting in increasingdegrees of chronic low-grade metabolic acidosis. The atten-dant increased blood acidity and hypobicarbonatemia pro-vide the proximate signals for a cell-mediated increase inbone turnover that contributes to the development of os-teoporosis and increases hip fracture risk in postmenopausalwomen. Moderation of animal food consumption and an in-creased ratio of vegetable/animal food consumption mayconfer a protective effect.AcknowledgmentsThis research was supported in addition by the National Institutes ofHealth (Grants M01 RR00079, R01 AG/AR 05407, P01DK 39964, andR01HL47943); the University of California Research Evaluations and Allo-cation Committee (Grant MSC-22); UCSF Academic Senate Grant; andgifts from Church & Dwight Co., Inc., and the Emil Mosbacher, Jr., Foun-dation. The authors thank Vivian Weinberg for her invaluable biostatisticalsupport and the UCSF General Clinical Research Center.Address correspondence to Dr. Anthony Sebastian, Box 0126, 1202Moffitt Hospital, University of California, San Francisco, CA 94143.E-mail: [email protected]References1. Abelow BJ, Holford TR, Insogna KL. Cross-cultural association be-tween dietary animal protein and hip fracture: a hypothesis.CalcifTissue Int.1992;50:14–18.2. Wachman A, Bernstein DS. Diet and osteoporosis.Lancet.1968;1:9518–959.
Figure 3. Cross-cultural relationship (n23 countries) between hip fracture incidence in Caucasian women aged 50 years and older (per100,000 person-years) and per capita animal protein (left panel) and vegetable protein consumption (right panel) (g/day).
DIET ACID LOAD AND HIP FRACTURE INCIDENCE
M591
3. Barzel US, Massey LK. Excess dietary protein can adversely affectbone.J Nutr.1998;128:1051–1053.4. Kleinman JG, Lemann J, Jr. Acid production. In: Maxwell MH, Klee-man CR, Narins RG, eds.Clinical Disorders of Fluid and ElectrolyteMetabolism.New York: McGraw Hill; 1987:159–173.5. Souci SW, Fachmann W, Kraut H.Food Composition and NutritionTables.Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH; 1986.6. Remer T, Manz F. Potential renal acid load of foods and its influenceon urine pH.J Am Diet Assoc.1995;95:791–797.7. Hu J-F, Zhao X-H, Parpia B, Campbell TC. Dietary intakes and uri-nary excretion of calcium and acids: a cross-sectional study ofwomen in China.Am J Clin Nutr.1993;58:398–406.8. Barber J, Mills H, Horne G, Purdie G, Devane P. The incidence of hipfractures in Maori and non-Maori in New Zealand.N Z Med J.1995;108:367–368.9. Coster A, Haberkamp M, Allolio B. Inzidenz von Schenkelhalsfrak-turen in der Bundesrepublik Deutschland im internationalenVergleich [Incidence of femoral neck fractures in the German FederalRepublic in comparison to other countries].Soz Praventivmed.1994;39:287–292.10. Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA.Symptomatic fracture incidence in elderly men and women: theDubbo Osteoporosis Epidemiology Study (DOES).Osteoporos Int.1994;4:277–282.11. Baudoin C, Fardellone P, Potard V, Sebert JL. Fractures of the proxi-mal femur in Picardy, France, in 1987.Osteoporos Int.1993;3:43–49.12. Fujiwara NK, Marti B, Gutzwiller F. Hip fracture mortality and mor-bidity in Switzerland and Japan: a cross-cultural comparison.SozPraventivmed.1993;38:8–14.13. Nakamura T. Epidemiological study on hip fractures in Tottori Pre-fecture [in Japanese].Nippon Seikeigeka Gakkai Zasshi.1993;67:189–200.14. Prince RL, Knuiman MW, Gulland L. Fracture prevalence in an Aus-tralian population.Aust J Public Health.1993;17:124–128.15. Vaz AL. Epidemiology and costs of osteoporotic hip fractures in Por-tugal.Bone.1993;14(suppl 1):S9.16. Dretakis EK, Giaourakis G, Steriopoulos K. Increasing incidence ofhip fracture in Crete.Acta Orthop Scand.1992;63:150–151.17. Ferrandez L, Hernandez J, Gonzalez-Orus A, Devesa F, Ceinos M.Hip fracture in the elderly in Spain. Incidence 1977–88 in the prov-ince of Salamanca.Acta Orthop Scand.1992;63:386–388.18. Olmos JM, Martinez J, Garcia J, Matorras P, Moreno JJ, Gonzalez-Macias J. Incidencia de fractura de cadera en Cantabria [Incidence ofhip fractures in Cantabria].Med Clin (Barc).1992;99:729–731.19. Adebajo AO, Cooper C, Evans JG. Fractures of the hip and distalforearm in West Africa and the United Kingdom.Age Ageing.1991;20:435–438.20. Bagur A, Rubin Z, Garcia M, Mautalen CA. Epidemiologia de lasfracturas del femur proximal en La Plata, Argentina [Epidemiologyof proximal femoral fractures in La Plata, Argentina].Medicina (BAires).1991;51:343–347.21. Contreras L, Kirschbaum A, Pumarino H. Epidemiologia de las frac-turas en Chile [Epidemiology of fractures in Chile].Rev Med Chil.1991;119:92–98.22. Martin AD, Silverthorn KG, Houston CS, Bernhardson S, Wajda A,Roos LL. The incidence of fracture of the proximal femur in two mil-lion Canadians from 1972 to 1984. Projections for Canada in the year2006.Clin Orthop.1991;266:111–118.23. Kellie SE, Brody JA. Sex-specific and race-specific hip fracture rates.Am J Public Health.1990;80:326–328.24. Lau EM, Cooper C, Wickham C, Donnan S, Barker DJ. Hip fracturein Hong Kong and Britain.Int J Epidemiol.1990;19:1119–1121.25. Ray WA, Griffin MR, West R, Strand L, Melton LJ. Incidence of hipfracture in Saskatchewan, Canada, 1976–1985.Am J Epidemiol.1990;131:502–509.26. Caniggia M, Morreale P. Epidemiology of hip fractures in Siena, It-aly, 1975–1985.Clin Orthop.1989;238:131–138.27. Diez A, Puig J, Martinez MT, Diez JL, Aubia J, Vivancos J. Epidemi-ology of fractures of the proximal femur associated with osteoporosisin Barcelona, Spain.Calcif Tissue Int.1989;44:382–386.28. Hagino H, Yamamoto K, Teshima R, Kishimoto H, Kuranobu K, Na-kamura T. The incidence of fractures of the proximal femur and the
29.30.31.32.33.34.35.36.37.38.39.40.41.42.43.44.45.46.
47.48.49.50.51.52.53.54.55.56.
distal radius in Tottori prefecture, Japan.Arch Orthop Trauma Surg.1989;109:43–44.Rodriguez JG, Sattin RW, Waxweiler RJ. Incidence of hip fractures,United States, 1970–83.Am J Prev Med.1989;5:175–181.Silverman SL, Madison RE. Decreased incidence of hip fracture inHispanics, Asians, and blacks: California Hospital discharge data.Am J Public Health.1988;78:1482–1483.Finsen V, Benum P. Changing incidence of hip fractures in rural andurban areas of central Norway.Clin Orthop.1987;218:104–110.Lizaur-Utrilla A, Puchades OA, Sanchez DC, Anta BJ, Gutierrez CP.Epidemiology of trochanteric fractures of the femur in Alicante,Spain, 1974–1982.Clin Orthop.1987;218:24–31.Makin M. Osteoporosis and proximal femoral fractures in the femaleelderly of Jerusalem.Clin Orthop.1987;218:19–23.Currie AL, Reid DM, Brown N. An epidemiological study of fractureof the neck of femur.Health Bull.1986;44:143–148.Hedlund R, Ahlbom A, Lindgren U. Hip fracture incidence in Stock-holm, 1972–1981.Acta Orthop Scand.1986;57:30–34.Barss P. Fractured hips in rural Melanesians: a nonepidemic.TropGeogr Med.1985;37:156–159.Boyce WJ, Vessey MP. Rising incidence of fracture of the proximalfemur.Lancet.1985;1:150–151.Falch JA, Ilebekk A, Slungaard U. Epidemiology of hip fractures inNorway.Acta Orthop Scand.1985;56:12–16.Luthje P. Incidence of hip fracture in Finland. A forecast for 1990.Acta Orthop Scand.1985;56:223–225.Farmer ME, White LR, Brody JA, Bailey KR. Race and sex differ-ences in hip fracture incidence.Am J Public Health.1984;74:1374–1380.Zain Elabdien BS, Olerud S, Karlstrom G, Smedby B. Rising inci-dence of hip fracture in Uppsala, 1965–1980.Acta Orthop Scand.1984;55:284–289.Zetterberg C, Elmerson S, Andersson GB. Epidemiology of hip frac-tures in Goteborg, Sweden, 1940–1983.Clin Orthop.1984;191:43–52.Frandsen PA, Kruse T. Hip fractures in the county of Funen, Den-mark. Implications of demographic aging and changes in incidencerates.Acta Orthop Scand.1983;54:681–686.Luthje P. Fractures of the proximal femur in Finland in 1980.AnnChir Gynaecol.1983;72:282–286.Swanson AJ, Murdoch G. Fractured neck of femur. Pattern of inci-dence and implications.Acta Orthop Scand.1983;54:348–355.Hoogendoorn D. Enkele gegevens over 64.453 fracturen van hetproximale uiteinde van het femur (collum plus trochantergebied),1967–1979 [Data on 64,453 fractures of the proximal end of the fe-mur (neck and trochanter area), 1967–1979].Ned Tijdschr Geneeskd.1982;126:963–968.Rees JL. Secular changes in the incidence of proximal femoral frac-ture in Oxfordshire: a preliminary report.Community Med.1982;4:100–103.Baker MR. An investigation into secular trends in the incidence offemoral neck fracture using hospital activity analysis.Public Health.1980;94:368–374.Gallagher JC, Melton LJ, Riggs BL, Bergstrath E. Epidemiology offractures of the proximal femur in Rochester, Minnesota.Clin Or-thop.1980;150:163–171.Jensen JS. Incidence of hip fractures.Acta Orthop Scand.1980;51:511–513.Matkovic V, Ciganovic M, Tominac C, Kostial K. Osteoporosis andepidemiology of fractures in Croatia. An international comparison.Henry Ford Hosp Med J.1980;28:116–126.Stott S, Gray DH. The incidence of femoral neck fractures in NewZealand.N Z Med J.1980;91:6–9.Evans JG, Prudham D, Wandless I. A prospective study of fracturedproximal femur: incidence and outcome.Public Health.1979;93:235–241.Evans JG. Fractured proximal femur in Newcastle upon Tyne.AgeAgeing.1979;8:16–24.Solomon L. Bone density in ageing Caucasian and African popula-tions.Lancet.1979;2:1326–1330.Colbert DS, O’Muircheartaigh I, Chater EH, Wilson AL, Moore A. Astudy of fracture of the neck of the femur in the west of Ireland 1968–1973.Ir Med J.1976;69:1–12.
M592
FRASSETTO ET AL.
57. Alhava EM, Puittinen J. Fractures of the upper end of the femur as anindex of senile osteoporosis in Finland.Ann Clin Res.1973;5:398–403.58. Chalmers J, Ho KC. Geographical variations in senile osteoporosis.The association with physical activity.J Bone Joint Surg Br.1970;52:667–675.59. Levine S, Makin M, Menczel J, Robin G, Naor E, Steinberg R. Inci-dence of fractures of the proximal end of the femur in Jerusalem. Astudy of ethnic factors.J Bone Joint Surg Am.1970;52:1193–1202.60. Solomon L. Osteoporosis and fracture of the femoral neck in theSouth African Bantu.J Bone Joint Surg Br.1968;50:2–13.61. Wong PC. Fracture epidemiology in a mixed southeastern Asiancommunity (Singapore).Clin Orthop.1966;45:55–61.62. Alffram P-A. An epidemiologic study of cervical and trochantericfractures of the femur in an urban population. Analysis of 1,664 caseswith special reference to etiologic factors.Acta Orthopaed Scand.1964(suppl 65):101–109.63. Knowelden J, Buhr AJ, Dunbar O. Incidence of fracture in personsover 35 years of age. A report to the M.R.C. working party on frac-tures in the elderly.Brit J Prev Soc Med.1964;18:130–141.64. Buhr AJ, Cooke AM. Fracture patterns.Lancet.1959;1:531–536.65. Suriyawongpaisal P, Siriwongpairat P, Loahachareonsombat W, et al.A multicenter study on hip fractures in Thailand.J Med Assoc Thai.1994;77:488–495.66. Rowe SM, Yoon TR, Ryang DH. An epidemiological study of hipfracture in Honam, Korea.Int Orthop.1993;17:139–143.67. Pedrazzoni M, Alfano FS, Malvi C, Ostanello F, Passeri M. Seasonalvariation in the incidence of hip fractures in Emilia-Romagna andParma.Bone.1993;14(suppl 1):S57–S63.68. Lee CM, Sidhu JS, Pan KL. Hip fracture incidence in Malaysia 1981–1989.Acta Orthop Scand.1993;64:178–180.69. Lau EM, Cooper C. Epidemiology and prevention of osteoporosis inurbanized Asian populations.Osteoporos Int.1993;3(suppl 1):23–26.70. Ho SC, Bacon WE, Harris T, Looker A, Maggi S. Hip fracture ratesin Hong Kong and the United States, 1988 through 1989.Am J PublicHealth.1993;83:694–697.71. Gullberg B, Duppe H, Nilsson B, et al. Incidence of hip fractures inMalmo, Sweden (1950–1991).Bone.1993;14(suppl 1):S23–S29.72. Agnusdei D, Camporeale A, Gerardi D, Rossi S, Bocchi L, GennariC. Trends in the incidence of hip fracture in Siena, Italy, from 1980 to1991.Bone.1993;14(suppl 1):S31–S34.73. Al-Nuaim AR, Kremli M, Al-Nuaim M, Sandkgi S. Incidence ofproximal femur fracture in an urbanized community in Saudi Arabia.Calcif Tissue Int.1995;56:536–538.74. Matkovic V, Kostial K, Simonovic I, Buzina R, Brodarec A, NordinBEC. Bone status and fracture rates in two regions of Yugoslavia.AmJ Clin Nutr.1979;32:540–549.75. Armitage P.Statistical Methods in Medical Research.New York:Blackwell Scientific; 1971.76.United States Population Estimates, by Age, Sex and Race: 1980 to1987.Washington, DC: US Department of Commerce, Bureau of theCensus; 1988:11–12. Series P-25 (No. 1022).77.Food Balance Sheets (1954–56; 1957–59; 1964–66; 1975–77; 1984–86).Statistics Division of the Economic and Social Policy Depart-ment. Rome, Italy: Food and Agriculture Organization of the UnitedNations; 1991.78. Halperin ML. Metabolism and acid-base physiology.Artif Organs.1982;6:357–362.79. Blatherwick NR. The specific role of foods in relation to the compo-sition of the urine.Arch Int Med.1914;14:409–450.80. Robertson WG, Peacock M, Heyburn PJ, et al. Should recurrent cal-cium oxalate stone formers become vegetarians?Br J Urol.1979;51:427–431.
81. Breslau NA, Brinkley L, Hill KD, Pak CYC. Relationship of animalprotein-rich diet to kidney stone formation and calcium metabolism.J Clin Endocrinol Metab.1988;66:140–146.82. Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A. Effect ofdiet on plasma acid-base composition in normal humans.Kidney Int.1983;24:670–680.83. Frassetto L, Morris RC, Jr., Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal func-tional decline.Am J Physiol.1996;271:1114–1122.84. Alpern RJ. Trade-offs in the adaptation to acidosis.Kidney Int.1995;47:1205–1215.85. Alpern RJ, Sakhaee S. The clinical spectrum of chronic metabolic ac-idosis: homeostatic mechanisms produce significant morbidity.Am JKid Dis.1997;29:291–302.86. Lemann J, Jr., Litzow JR, Lennon EJ. The effects of chronic acidloads in normal man: further evidence for participation of bone min-eral in the defense against chronic metabolic acidosis.J Clin Invest.1966;45:1608–1614.87. Bushinsky DA. Internal exchanges of hydrogen ions: bone. In: SeldinDW, Giebisch G, eds.The Regulation of Acid-Base Balance.NewYork: Raven Press; 1989:69–88.88. Lemann J, Jr., Lennon EJ, Goodman AD, Litzow JR, Relman AS.The net balance of acid in subjects given large loads of acid or alkali.J Clin Invest.1965;44:507–517.89. Lemann J, Jr., Litzow JR, Lennon EJ. Studies of the mechanism bywhich chronic metabolic acidosis augments urinary calcium excre-tion in man.J Clin Invest.1967;46:1318–1328.90. Barzel US. The role of bone in acid-base metabolism. In: Barzel US,ed.Osteoporosis.New York: Grune & Stratton; 1970:199–206.91. Barzel US. The effect of excessive acid feeding on bone.Calcif TissRes.1969;4:94–100.92. Barzel US. Acid-induced osteoporosis: an experimental model of hu-man osteoporosis.Calcif Tiss Res.1976;21(suppl):417–422.93. Barzel US, Jowsey J. The effects of chronic acid and alkali adminis-tration on bone turnover in adult rats.Clin Sci.1969;36:517–524.94. Jaffe HL, Bodansky A, Chandler JP. Ammonium chloride decalcifi-cation, as modified by calcium intake. The relation betweengeneralized osteoporosis and osteitis fibrosa.J Exp Med.1932;56:823–834.95. Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits osteoblas-tic and stimulates osteoclastic activity in vitro.Am J Physiol.1992;262:F442–F448.96. Lennon EJ, Lemann J, Jr., Litzow JR. The effect of diet and stoolcomposition on the net external acid balance of normal subjects.JClin Invest.1966;45:1601–1607.97. Green J, Kleeman CR. The role of bone in regulation of systemicacid-base balance.Kidney Int.1991;39:9–26.98. Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC, Jr. Im-proved mineral balance and skeletal metabolism in postmenopausalwomen treated with potassium bicarbonate [see comments].N Engl JMed.1994;330:1776–1781.99. Massey LK. Does excess dietary protein adversely affect bone? Sym-posium overview.J Nutr.1998;128:1048–1050.100. Heaney RP. Excess dietary protein may not adversely affect bone.JNutr.1998;128:1054–1057.101. Willett W.Nutritional Epidemiology.New York: Oxford UniversityPress; 1998.
Received July 13, 1999Accepted December 23, 1999Decision Editor: William B. Ershler, MD